Inovotion has developed an in ovo model to assess the activity of immuno-oncology drug candidates. This technique induces the complete native chick immune system in response to grafting of human cancer cells and has great potential to significantly reduce the use of humanised mouse models in the preclinical testing of anticancer compounds. Academic and/or industry partners are sought to provide diverse, novel immuno-oncology compounds (with preclinical data available) to test in this new model.
The development of efficient new drugs in immuno-oncology (IO) is limited by the availability and cost of reliable in vivo models. The current models used are humanised mice or syngeneic mouse tumour models, but the clinical predictivity of these models is subject to controversy (Yong, Her and Chen, 2018). In humanised mice, secondary lymphoid structures are either missing or disorganised; this curtails essential humoral responses, resulting in impairments for both immunoglobulin class-switching and affinity maturation post-immunisation (Yong, Her and Chen, 2018). Additionally, an absence of essential human cytokines hinders optimal hematopoietic stem cell (HSC) engraftment, differentiation, and maturation of functional immune cells (Yong, Her and Chen, 2018). These limitations reduce the applicability of the mouse model, because the reconstituted humanised immune system is not comparable physiologically to the human patients.
The generation of humanised mice for immuno-therapy models takes approximately three months and the procedure is rated as “moderate” severity (Ec.europa.eu, 2018). Firstly, the animal’s immune system is depleted by irradiation, which is followed by the graft of human immune precursor cells and several weeks in a sterile environment to enable the human immune system to become established. Tumour cell xenografts are then introduced, and finally the IO anticancer treatments can be evaluated.
Thus, there is a need to develop fast, sensitive, and reliable 3Rs-compliant IO models allowing large in vivo screenings for various types of IO compounds including immune checkpoint inhibitors, vaccines or adoptive cell therapies.
References
- Ec.europa.eu. (2018). Expert working group on severity classification of scientific procedures performed on animals. Available at: http://ec.europa.eu/environment/chemicals/lab_animals/pdf/report_ewg.pdf [Accessed 20 Dec. 2018].
- Yong K, Her Z, and Chen Q (2018). Humanized Mice as Unique Tools for Human-Specific Studies. Archivum Immunologiae et Therapiae Experimentalis 66(4): 245-266. doi: 10.1007/s00005-018-0506-x
Inovotion has developed a new assay for IO drug screening that is sensitive and reliable, using embryonated chicken eggs with a complete immune system, to test the efficacy and toxicity of anticancer drug candidates in the same in ovo model, rather than two mouse models. This in ovo model generates faster results, offers increased statistical power, higher sensitivity, and reduced costs in comparison to the mouse model [Annex 1]. This technique has great potential for significantly reducing the use of animals in preclinical testing of anticancer compounds (Gilson et al., 2017; Guedat et al., 2017; El Hasasna et al., 2016; Kroiss et al., 2014; Al Dhaheri et al., 2013; Mikaelian et al., 2013; Prudent et al., 2013).
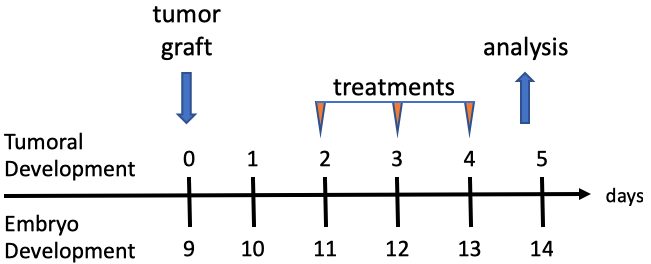
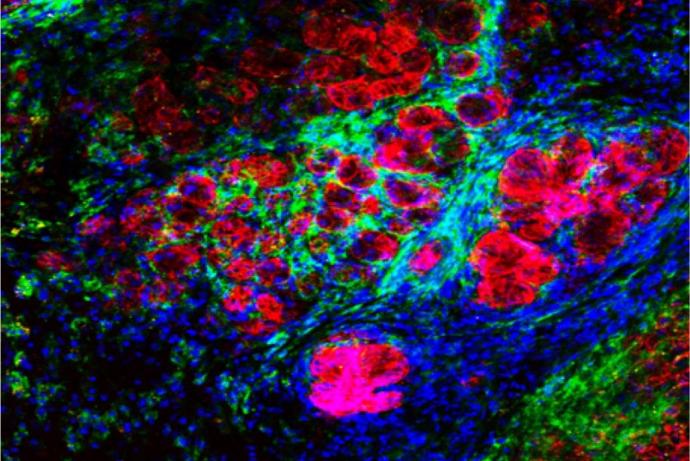
The assay is based on the company’s previous chick embryo model and has undergone further development to induce the complete immune system at an earlier timepoint. Human cancer cells or patient derived xenografts are grafted on the chick embryo’s chorioallantoic membrane (CAM), the tumours are cultured for four days and harvested at day 13.5 of egg incubation (Figure 1). Tumour growth follows the rapid but physiological growth of the embryo, and the tumours obtained are of a comparable size to the mouse tumours and infiltrated with immune cells (lymphocyte-T, natural killers, and macrophages) [Annex 2]. During this four-day period, it is possible to treat the embryo with IO compounds while the tumour is growing and evaluate the effects of the treatment on tumour growth, metastatic invasion of the organs and lower CAM, and tumoural infiltration by immune cells, in comparison with reference molecules.
Figure 1. In ovo model experiment timeline.
Inovotion believe they have developed a more physiologically relevant model, which is more predictive of results in humans. This is primarily because the chick immune system is native and contains similar populations of immune cells and molecular mechanisms as are found in human patients, according to Inovotion’s internal R&D results (can be shared upon request). The innate immune response (the leukocyte population including macrophages and natural killer cells) exists at the time of the graft, and the adaptive immune system (including T-cell antigen presentation, B-cell response, antibody production) becomes mature and effective at the time of treatment with immuno-oncology anticancer compounds.
The in ovo model has been tested with three immune checkpoint inhibitors (one PD-1 inhibitor and two PDL-1 inhibitors) available on the market [Annex 1-3]. The model can be used for performing large in vivo screening studies (approximately 100 experimental conditions/ compounds simultaneously) of various IO compounds including immune checkpoint inhibitors, vaccines or adoptive cell therapies. This new approach can improve lead optimisation of anticancer IO compounds; measuring quantitatively and simultaneously the impact of the drug on tumour growth and metastatic invasion as well as the toxicity of lead candidates.
References
- Al Dhaheri Y, Attoub S, Arafat K, et al. (2013). Anti-Metastatic and Anti-Tumor Growth Effects of Origanum majorana on Highly Metastatic Human Breast Cancer Cells: Inhibition of NFκB Signaling and Reduction of Nitric Oxide Production. PLoS ONE 8(7): e68808. doi: 10.1371/journal.pone.0068808
- El Hasasna H, Saleh A, Samri H, et al. (2016). Rhus coriaria suppresses angiogenesis, metastasis and tumor growth of breast cancer through inhibition of STAT3, NFκB and nitric oxide pathways. Scientific Reports. 6(1): 21144. doi: 10.1038/srep21144
- Gilson P, Josa-Prado F, Beauvineau C, et al. (2017). Identification of pyrrolopyrimidine derivative PP-13 as a novel microtubule-destabilizing agent with promising anticancer properties. Scientific Reports 7(1): 10209. doi: 10.1038/s41598-017-09491-9
- Guedat P, Miniou P (2017). Method of treating cancer with a combination of benzylideneguanidine derivatives and chemotherapeutic agent. Patent N° WO2017/021216 A1.
- Kroiss A, Vincent S, Decaussin-Petrucci M, et al. (2014). Androgen-regulated microRNA-135a decreases prostate cancer cell migration and invasion through downregulating ROCK1 and ROCK2. Oncogene 34(22): 2846-2855. doi: 10.1038/onc.2014.222
- Mikaelian I, Malek M, Gadet R, et al. (2013). Genetic and Pharmacologic Inhibition of mTORC1 Promotes EMT by a TGF beta Independent Mechanism. Cancer Research 73(22): 6621-6631. doi: 10.1158/0008-5472.CAN-13-0560
- Prudent R, Vassal-Stermann É, Nguyen C, et al. (2013). Azaindole derivatives are inhibitors of microtubule dynamics, with anti-cancer and anti-angiogenic activities. British Journal of Pharmacology 168(3): 673-685. doi: 10.1111/j.1476-5381.2012.02230.x
Inovotion has developed IO tests based on three human tumour cell lines including breast, lymphoma and lung, with a limited number of commercial IO drugs: Keytruda and Tecentriq (Annexes1, 2 and 4). To complete these validation studies and demonstrate the utility of the approach over humanised mouse models, it is important to measure the activity and toxicity of a larger set of IO anticancer drugs.
Inovotion seek industry or academic partners able to provide diverse novel IO reagents or compounds (i.e. small molecules, antibodies, modified T-cells) to test in the in ovo model. These compounds can be marketed or in development and should have preclinical/clinical data available to enable the results obtained to be validated against existing mouse/ human data.
Inovotion offer early and privileged access to the technology, as well as the opportunity for a medium to long-term collaboration to set up dedicated models. The results of this further development/ validation work would be jointly published with any partners, whilst keeping the nature of the molecules confidential. Inovotion would also welcome input from the partners to improve the model according to their needs.
Information on IP
Inovotion’s basic technology is protected (patent pending). Specific patents are pending for the new Immuno-Oncology testing technology. Inovotion intend to extend this protection to all new technology based on it. Nonetheless, the company’s policy is to share IP rights where appropriate with their partners, each within their own domain of specialisation.
The generation of humanised mice for immuno-therapy models takes three months and is of moderate severity. For this procedure the animals are generally singularly housed for the three-month duration, to maintain the sterile environment required for this process. This introduces considerable stress and anxiety, negatively impacting the animal’s welfare, which in turn can confound results (Olsson et al., 2007). This in ovo model has a strong potential to replace the use of humanised mice, avoiding these welfare issues.
In the UK, in 2017 there were 108,000 mice used in oncology studies (UK Home Office, 2018). Inovotion estimate that approximately 36,000 of these are humanised mice and 36,000 are standard mice dedicated to immune-oncology studies, which is based on the fact that 60-70% of new molecules in oncology clinical trials are immune-oncology (Clinicaltrials.gov, 2019). For efficacy testing, each IO compound requires six to 12 humanised mice per group, and at least five groups, so 30 to 60 mice in total. Toxicity testing does not require humanised mice but uses the same experimental design so 30 to 60 additional mice are needed for these studies too. Therefore 60 to 120 mice are used to assess the efficacy and toxicity of each IO compound, and these could be replaced using Inovotion’s in ovo model. By pre-screening the IO treatments using the in ovo model, it allows researchers to test only promising high-value compounds on mice, to meet the requirements of regulatory agencies such as the FDA and EMA. The percentage of compounds that will show toxicity or poor efficacy in the in ovo screen cannot currently be precisely predicted but there is potential to reduce the number of animals by many hundreds.
References
- Clinicaltrials.gov. (2019). Home - ClinicalTrials.gov. [online] Available at: https://clinicaltrials.gov/ [Accessed 7 May 2019].
- Olsson IAS, Westlund K (2007). More than numbers matter: The effect of social factors on behaviour and welfare of laboratory rodents and non-human primates. Appl Anim Behav Sci 103(3-4):229–254. doi: 10.1016/j.applanim.2006.05.022
- UK Home Office (2018). Annual Statistics of Scientific Procedures on Living Animals Great Britain 2017. UK National Statistics