Galleria mellonella larvae have been shown to have utility for research into microbial infection, for antimicrobial drug screening, to test the toxicity of chemicals and to understand the host response to infection. TruLarv™ are research-grade G. mellonella larvae developed by BioSystems Technology Ltd. Partners are sought to explore the integration of TruLarv™ into drug screening platforms. TruLarv™ could be used for early stage toxicity or efficacy screening to identify candidates for further testing in mammals. TruLarv™ could also have applications in related areas such as chemical toxicity testing and the testing of environmental samples.
BioSystems Technology Ltd. partnered with Demuris and Envigo (now Covance CRS) through CRACK IT Solutions, and received funding for two projects. The collaboration with Demuris aims to validate the TruLarv model as a screen for crude actinomycete extracts for potential new antimicrobial drugs. The partnership with Envigo aims to compare acute oral toxicity testing of 25 compounds in G. mellonella with existing data from cell culture methods.
These projects and their outputs are featured as a case study in the 2019 CRACK IT Review.
Attrition rates in drug discovery programmes are a problem for the pharmaceutical industry, adding cost and time to drug development programmes. Failures of drugs to perform as expected, because of toxicity or lack of efficacy, often appear during costly human clinical studies highlighting the lack of translation of current preclinical models. There is an urgent need for models that bridge the gap between in vitro studies and mammalian studies to improve the predictivity of preclinical studies and reduce late stage attrition.
G. mellonella larvae are living organisms that have been used widely in the past five years as microbial infection models (Tsai et al., 2016); antimicrobial drug screening models (Desbois and Coote, 2012); models to test the toxicity of chemicals (Megaw et al., 2015); and models to understand the host response to infection (Harding et al., 2013). The major advantages of G. mellonella larvae over other invertebrate models are the ability to carry out experiments at 37°C and to precisely dose individual larvae with drugs/microorganisms by injection into a defined site. Reported studies include the testing of over 30 antibiotics and antifungal compounds in Galleria for their abilities to control infections caused by a range of pathogens and there is good agreement between the results of studies in humans and in Galleria (Thomas et al., 2013).
More recently there has been a shift towards using this model to screen new approaches to disease control. These studies range from the testing of novel combinations of existing drugs to the testing of completely new treatments including the use of nanoparticles to deliver drugs (Deacon et al., 2015), bacteriophage therapies (Seed and Dennis, 2009), chemicals such as gallium, silver and zinc (Antunes et al., 2012; Coughlan et al., 2010), antimicrobial peptides (Dean et al., 2011) and photodynamic therapy (Chibebe et al., 2013). The diverse range of approaches to disease control, which have been tested at an early stage, indicate the potential value of Galleria as an in vivo screening model.
However, the G. mellonella model has not been adopted outside of academia because the larvae used are typically sold as feedstuffs for pet reptiles and fish. Whilst cheap to purchase, there are significant variations in the behaviour of larvae within and between batches (Tsai et al., 2016). This is a barrier to the further development and wider adoption of this model.
References
- Antunes LC, Imperi F, Minandri et al. (2012). In vitro and in vivo antimicrobial activities of gallium nitrateagainst multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 56(11): 5961-70. doi:10.1128/AAC.01519-12.
- Chibebe Junior J, Fuchs BB, sabino CP, et al. (2013). Photodynamic and antibiotic therapy impair the pathogenesis of Enterococcus faecium in a whole animal insect model. PLoS One 8(2): e55926. doi:10.1371/journal.pone.0055926.
- Coughlan A, Scanlon K, Mahon BP, et al. (2010). Zinc and silver glass polyalkenoate cements: an evaluation of their antibacterial nature. Biomed Mater Eng 20(2): 99-106. doi:10.3233/BME-2010-0620.
- Deacon J, Abdelghany SM, Quinn DJ, et al. (2015). Antimicrobial efficacy of tobramycin polymeric nanoparticles for Pseudomonas aeruginosa infections in cystic fibrosis: formulation, characterisation and functionalisation with dornase alfa (DNase). J Control Release 198: 55-61. doi:10.1016/j.jconrel.2014.11.022.
- Dean SN, Bishop BM and van Hoek ML (2011). Susceptibility of Pseudomonas aeruginosa Biofilm to Alpha-Helical Peptides: D-enantiomer of LL-37. Front Microbiol 2: 128. doi:10.3389/fmicb.2011.00128.
- Desbois AP and Coote PJ (2012). Utility of Greater Wax Moth Larva (Galleria mellonella) for Evaluating the Toxicity and Efficacy of New Antimicrobial Agents. Adv Appl Microbiol 78: 25-53. doi:10.1016/B978-0-12-394805-2.00002-6.
- Harding CR, Stoneham CA, Schuelein R, et al. (2013). The Dot/Icm effector SdhA is necessary for virulence of Legionella pneumophila in Galleria mellonella and A/J mice. Infect Immun 81(7): 2598-605. doi:10.1128/IAI.00296-13.
- Megaw J, Thompson TP, Lafferty RA, et al. (2015). Galleria mellonella as a novel in vivo model for assessment of the toxicity of 1-alkyl-3-methylimidazolium chloride ionic liquids. Chemosphere 139: 197-201. doi:10.1016/j.chemosphere.2015.06.026.
- Seed KD and Dennis, JJ (2009). Experimental bacteriophage therapy increases survival of Galleria mellonella larvae infected with clinically relevant strains of the Burkholderia cepacia complex. Antimicrob Agents Chemother 53(5): 2205-8. doi:10.1128/AAC.01166-08.
- Thomas RJ, Hamblin KA, Armstrong SJ, et al. (2013). Galleria mellonella as a model system to test the pharmacokinetics and efficacy of antibiotics against Burkholderia pseudomallei. Int J Antimicrob Agents 41(4): 330-6. doi:10.1016/j.ijantimicag.2012.12.009.
- Tsai CJ, Loh JM, and Proft T (2016). Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 1-16. doi:10.1080/21505594.2015.1135289.
TruLarv™ are research grade G. mellonella larvae developed by BioSystems Technology Ltd. TruLarv™ are weight and age defined, surface decontaminated and are bred without the use of antibiotics or other drugs. The genome sequence of the breeding colony is currently being determined. G. mellonella assays often use mortality as an endpoint, usually within 24 – 72 hours. However, disease progression in G. mellonella larvae can be scored on a numerical scale on the basis of change in mobility and colour, allowing simple and rapid scoring of test groups (Tsai et al., 2016). Scoring of larvae is currently performed by an individual at a series of defined timepoints.
End points in G. mellonella infection models include survival rate, which can be assessed up to five days post infection, facilitating the calculation of a maximum half lethal dose (LD50); expression of antimicrobial proteins in response to infection; production of lactate dehydrogenase as a marker of cell damage and biophotonic imaging to measure proliferation of bioluminescent microorganisms responsible for larval infection. A pathological scoring system was recently proposed in which an assessment of larval mobility, cocoon formation, melanisation and survival was used to assess larval health.
BioSystems Technology Ltd have shown that TruLarv™ behave more consistently and reproducibly than pet shop larvae allowing the statistical power of experiments to be markedly increased. In some studies differences between test and control groups were not apparent using pet shop larvae, but were statistically different using TruLarv™ (data not published). These findings indicate the value of TruLarv™ and now open opportunities for this model to be developed for studies which bridge the gap between in vitro studies and mammalian studies. In addition, the potential to carry out high throughput studies now opens new opportunities to improve the effectiveness of drug discovery programmes.
This Solution is initially targeted towards organisations involved in the development of new antimicrobial drugs. TruLarv™ can be used to test compounds at an early stage both for toxicity and efficacy. However, it is envisaged that the larvae would have applications in related areas, such as the testing of drugs that modulate immune responses. The model would also have applications for chemical toxicity testing including the testing of environmental samples.
Reference
- Tsai CJ, Loh JM, and Proft T (2016). Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 1-16. doi: doi:10.1080/21505594.2015.1135289.
BioSystems Technology Ltd seek partners who are interested in integrating TruLarv™ into antimicrobial drug discovery platforms. TruLarv™ could be used at an early stage for toxicity and or efficacy screening, to identify candidates that would be suitable for progression through the drug development pathway for further testing studies in mammals. Collaborators with expertise in imaging and tracking technologies are also sought, to automate the scoring of large groups of larvae, allowing high throughput screens to be developed.
For these collaborations BioSystems Technology Ltd would contribute research grade larvae, expertise in handling larvae, and expertise in scoring disease in larvae. Partners who could provide compounds for efficacy or toxicity testing are sought and those who have an interest in integrating the model into test platforms in an industrial setting.
Information about IP
A patent application to protect breeding, colony management and quality control processes by BioSystems Technology Ltd has been filed.
Used as a preliminary screen, G. mellonella larvae facilitate the selection of promising leads for further testing in vertebrates, significantly reducing the number of vertebrate animals used in drug development. For example, use of G. mellonella larvae early in the development of probiotics has reduced the use of vertebrate model hosts (poultry) by 80% in some studies. Around 50 lactobacilli strains have been screened in the Galleria model in order to select 10 for further studies. These studies would normally be carried out in day old chicks, with five chicks being commonly used per lactobacilli strain. Using Galleria, it has been possible to reduce the number of chicks required for these studies by at least 200 (data not published – personal communication from Professor Roberto La Ragione, University of Surrey).
BioSystems Technology Ltd. have recieved CRACK IT Solutions funding for two projects to partner with Demuris and Envigo.
Demuris
Overview
Through CRACK IT Solutions, BioSystems Technology Ltd partnered with Demuris, an antibiotic discovery company who have access to a large, unique collection of actinomycete bacteria which are thought to produce completely novel antibacterial drugs. BioSystems Technology Ltd has been awarded funding through CRACK IT Solutions to work with Demuris to utilise the TruLarv model to screen crude actinomycete extracts for potential new antimicrobial drugs. Successful validation of this approach as an early toxicity and efficacy screen will enable rapid selection of promising leads and ensure poorly efficacious compounds are not progressed to animal studies.
Impact
In this proof-of-principle study, fifty crude actinomycete extracts which already show some antimicrobial activity on agar and potentially novel modes-of-action were selected from the Demuris collection. These crude extracts have unknown toxicity and efficacy profiles in vivo, data which is essential for Demuris to select the most promising lead targets. In this study the utility of G. mellonella as a screening tool for predicting efficacy and toxicity profiles in these extracts has been assessed.
In vivo toxicity measurements of complex bacterial extracts in TruLarv™
TruLarv™ was used as an early screen to evaluate complex bacterial extracts for acute toxicity. Forty one crude extracts (CE1-41) were screened individually in groups of five TruLarv™ in an acute toxicity assay, with larval survival scored 24 hours, 48 hours and 72 hours post challenge, used as the endpoint.
In vivo efficacy measurements of complex bacterial extracts in TruLarv™
Extracts had previously been screened for Gram-negative and Gram-positive antimicrobial activity in vitro using disc diffusion assays. Initially seven crude extracts were selected for further screening to test for in vivo antibacterial activity against the Gram-negative pathogen Pseudomonas aeruginosa (CE29, CE30 and CE31) and the Gram-positive pathogen Staphylococcus aureus (CE38, CE39, CE40 and CE41). To determine the in vivo activity of the extracts larvae were concurrently dosed with either 10μl of P. aeruginosa or S. aureus in one proleg and 10μl of crude extract in another pro leg. Larval survival was measured at 19 hours post- infection. Of the three crude extracts that were tested, CE29 and CE30 showed antimicrobial activity against P. aeruginosa and extract CE39 and CE40 showed activity against S. aureus.
Based on the initial screening a number of extracts were chosen for compound identification and dereplication by several rounds of chromatography and fractionation and subsequent LC-MS analysis. This process identified that extract CE41 contained the broad spectrum antibiotic paromomycin, demonstrating the potential of the G. mellonella model for identifying important antibiotics. The identification and dereplication of the active compounds from the other extracts is on-going.
Using the same compound identification and dereplication process, it was discovered that extract CE30 (which showed antimicrobial activity against P. aeruginosa) produces a novel potentially antimicrobial compound. Furthermore, cytotoxicity data from cell culture assays correlate with the data generated in TruLarvTM, further demonstrating the potential of the G. mellonella model in identifying novel antimicrobial products and accurately predicting efficacy and toxicity profiles.
The use of TruLarv in the early stage pipeline will enable the selection of promising leads for further testing in vertebrates, significantly reducing the number of vertebrate animals used in drug development. For example, a PK/PD study for a single compound using 14 doses and three mice per dose would require 42 mice plus the control group of six mice (total 48 mice per study). On average two PK/PD studies are carried out per year at Demuris, so by using TruLarv it is therefore possible to replace almost 100 animals a year for these studies. For toxicity studies with four doses of compound and three mice per dose would require 12 mice, plus the six mice for the control group, totals 18 mice per study. On average five toxicity studies are carried out per year at Demuris, so by using TruLarv™ it is therefore possible to replace nearly 200 animals a year for these studies
Dr Nick Allenby, CSO at Demuris Ltd said: “Through this NC3Rs-funded project Demuris has been able to use G. mellonella as a model system for the early stage toxicity evaluation of antibiotic compounds produced by actinomycete bacteria. The model enabled us to gain valuable early toxicity measurements allowing us to prioritise strains of interest for further development. Following on from this project Demuris will continue to use the model for toxicity evaluation and we are now using G. mellonella to evaluate the efficacy of our antibiotic compounds against a range of bacterial pathogens. Being able to prioritise compounds in this way will allow us to make better go/no-go decisions and reduce the number of compounds entering regulatory animal studies.”
To find out more about TruLarvTM G. mellonella visit Biosystems website
Presentations
- The results were presented as a talk at the Microbiology Society conference in Liverpool in April 2017,
Poster
- The results were presented as a poster at the European Pharmaceutics conference in Krakow in April 2017.
Envigo
Overview
With the support of funding from CRACK IT Solutions, the Exeter-based BioSystems Technology team is working with Envigo to compare acute oral toxicity testing of 25 compounds in G. mellonella with existing data from cell culture methods. The aim is to assess whether TruLarv provides a more accurate predictor of the mammalian LD50 than cell culture IC50 measurements.
If data obtained with TruLarv is more predictive of mammalian toxicity than existing cell culture methods, TruLarv toxicity testing will be offered as a service from Envigo, enabling wider uptake of the model by industry and reducing animal use by facilitating selection of only the most promising leads.
Impact
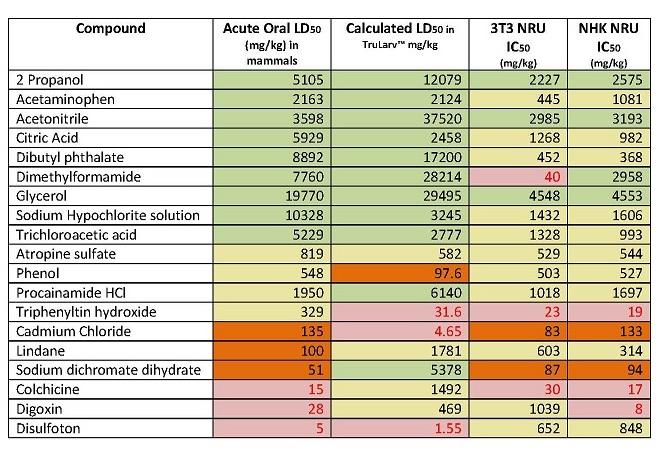
The purpose of this study was to investigate the suitability of TruLarv™ (larvae of the wax moth Galleria mellonella) as a model host for assessing acute toxicity of chemicals. 30 chemicals were assessed in the study, with results compared against IC50 values derived from two different in vitro cytotoxicity test methods (3T3 NRU and NK NRU assays, published in the OECD 129 guidance document) and in vivo acute oral LD50 values from rodents.
Of the 30 chemicals, it was possible to calculate the LD50 values for 19 of them. Due to solubility issues, LD50 values had to be estimated for eight chemicals. The remaining three chemicals could not be dosed in the TruLarvTM model due to lack of solubility in available solvents. Treatment was performed by subcutaneous microinjection of 10 µL test chemical into a defined injection site (front proleg). All treated and untreated control larvae were incubated at 20 - 25°C and scored for morbidity at 24 hours, 48 hours and 72 hours post challenge and LD50 (mg/kg) values in TruLarv™ calculated.
Categorising the hazard class of TruLarv™ LD50 values was based on the Globally Harmonized System (GHS) for classification and labelling of chemicals:
Category 5: >2000 mg/kg, non-toxic
Category 4: >300 - 2000 mg/kg, weakly toxic
Category 3: >50 - 300mg/kg, moderate - very toxic
Category 1-2: < 50 mg/kg, toxic
TruLarvTM accurately predicted 100% of the chemicals (nine out of nine) classified as non-toxic (category 5) based on published acute oral LD50 values. In comparison, cell-based cytotoxicity methods had only 44% concordance (four out of nine) with the acute oral toxicity method, with the remaining five chemicals being predicted as weakly toxic or very toxic (category 1 – 4). For one chemical (dimethylformamide) the IC50 values in the two in vitro tests were wildly different, with the 3T3 NRU assay classifying the chemical as category 1-2, and the NK NRU assay as category 5.
TruLarvTM correctly predicted 80% of the remaining ten chemicals as being in category 1-4, as defined by their acute oral LD50 values. However it was less accurate at determining which specific category these chemicals fell in to. In comparison, cytotoxicity methods predicted 100% of chemicals as being classified in category 1 – 4, but again were limited in predicting a specific hazard category.
For full results, see Table 1 below.
Table 1: Concordance of acute oral LD50 values categorised using the GHS with hazard categories predicted by TruLarv™ and cell-based cytotoxicity assays.
The potential for current cell-based cytotoxicity assays to identify false positive results triggers further regulatory in vivo studies. The results obtained in this study give an indication that the TruLarvTM model is potentially superior to these in vitro methods for predicting low-toxicity chemicals. Addition of this model to European Chemicals Agency (ECHA) guidance as a recommended element in a weight of evidence approach could strengthen the options for registrants to justify the waiving of in vivo acute oral toxicity studies for chemicals predicted to be of low toxicity and lead to a reduction in numbers of animals used for toxicity testing. For example, ECHA has estimated that approximately 550 in vivo acute oral studies could potentially be waived for chemicals that are due to be registered to meet the REACH 2018 deadline.
Specifically, the 3Rs impact of a reliable ECHA approved non-animal technology (NAT) predictor of low chemical toxicity would potentially reduce the numbers of OECD 420 Acute Oral Toxicity studies required for chemical registration. OECD 420 requires a minimum of five animals per study. Approximately 150 OECD 420 studies were performed at Envigo in 2017 using approximately 900 animals. 95% of these animals had a mild severity rating indicating that in general the chemicals tested were of low toxicity. Based on the premise that TruLarv was part of an ECHA approved integrated WoE approach, it is estimated that the animal testing requirement could potentially be reduced by up to 50% (approximately 450 animals) following justified waiver of testing.
Publications
- Allegra E et al. (2018). Galleria mellonella larvae allow the discrimination of toxic and non-toxic chemicals May. Chemosphere, 198: 469-472, doi:10.1016/j.chemosphere.2018.01.175.
Presentations
- The project results were presented as a talk at the Model Hosts Conference in Rhodes in September 2017.