Sirius Scissor is the first analytical instrument that successfully mimics the subcutaneous environment of the human body, in vitro, regarding its physical, chemical, and physiological parameters. It allows for the establishment of in vitro to in vivo correlations (IVIVC), formulation performance analysis (e.g. diffusion of components from the injection site and onset time) and determination of the stability upon injection of a formulation. The technology will enable early identification of formulations with poor performance or stability upon injection so that they can be adapted before they progress to animal studies. Sirius Analytical are seeking partners with access to compound and formulation information (including existing preclinical/clinical data) that can be used for further validation of the instrument.
New preparations intended for subcutaneous delivery are subjected to a battery of pharmacological tests to determine interaction between the active pharmaceutical ingredients (API), the formulation, and the subcutaneous space (Bhattachar et al., 2015). In vivo or ex vivo studies (often using rodents) are commonly used for this but have a number of recognised limitations. For example, though the studies can provide important information about the pharmacokinetics of a formulation, it is much more challenging to gather data regarding its stability and its particularities (e.g. API vs excipient diffusion) as these cannot be directly measured in vivo once a formulation has been injected. The poor correlation between animal models and the human response is being increasingly recognised, underlining the need for a new alternative to the current methods (Bracken 2009; Musther et al., 2014).
The scientific community is continuously looking for innovative ways to analyse compounds and formulations in a physiological and biorelevant environment. Sirius Scissor focuses on a different approach by mimicking in vitro the subcutaneous space of the human body. The goal is to evaluate and understand how a subcutaneous formulation performs after injection regarding its stability, diffusion of API and excipients from the injection site and uptake by the blood and lymphatic streams.
References
- Bhattachar SN, Bender DM, Sweetana SA et al. (2015). Discovery Formulations: Approaches and Practices in Early Preclinical Development. In Discovering and Developing Molecules with Optimal Drug-Like Properties, AAPS Advances in the Pharmaceutical Sciences Series volume 15 (Ed. Templeton AC, Byrn SR, Haskell RJ, Prisinzano TE), Springer, New York, NY. doi:10.1007/978-1-4939-1399-2_2.
- Bracken M (2009). Why animal studies are often poor predictors of human reactions to exposure. Journal of the Royal Society of Medicine 102(3):120-122. doi:10.1258/jrsm.2008.08k033.
- Musther H, Olivares-Morales A, Hatley OJD, et al. (2014). Animal versus human oral drug bioavailability: Do they correlate? European Journal of Pharmaceutical Sciences 57(100): 280-291. doi:10.1016/j.ejps.2013.08.018.
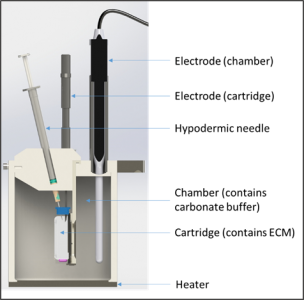
Sirius Scissor is the first scientific instrument available on the market that can successfully mimic the human subcutaneous space in vitro, using only synthetic materials. Sirius Scissor contains a cartridge (representing the injection site in the human body) filled with an artificial extra-cellular matrix (ECM) whose composition is similar to the human subcutaneous space. The cartridge is suspended in a chamber (containing a buffer similar to the interstitial fluid) and the formulation components (API and excipients) diffuse through a customised membrane on the cartridge into the chamber which can be seen in Figure 1. The membrane does not have a molecular weight cut-off but instead has pores with the same size and spacing as that of the blood and lymphatic vessels in the subcutaneous area.
Figure 1. Schematic of the Scissor components within the system.
Samples can also be withdrawn from the chamber at various time intervals throughout the experiment in order to perform offline protein quantification to calculate formulation performance. By using quantification techniques such as HPLC, the API of the formulation can be isolated and the profile monitored for the duration of the experiment. From this, the onset time of the drug (time taken for the API to begin entering the ‘blood stream’) can be determined as well as the release profile of the formulation from the site of injection into the simulated blood stream.
Sirius Scissor can be used throughout different stages of subcutaneous product development, but primarily for preclinical development (lead molecule selection and formulation screening). Sirius Analytical believe that this instrument can also provide valuable information on the medium and long term stabilities of a product. One can analyse the formulation performance over time and correlate that information with degradation events that occur under stress conditions.
All the components of the system are customisable (i.e. ECM, cartridge membrane, chamber buffer, temperature, pH) so it is also suitable for smaller studies; to understand more specific interactions and effects between the components of the subcutaneous space and the formulations analysed.
Scissor has been validated with antibody formulations (kindly supplied by Genentech) and allows for an IVIVC of 90% regarding the formulations’ bioavailability (Kinnunen et al., 2015). Additionally, Sirius Analytical have worked with two different insulin formulations (from Sanofi) which also show a very high correlation between human data (Insulin profile, Diabetes UK, n.d.) and that obtained using this novel technology.
References
- Diabetes UK. Insulin profiles PDF. Available at www.diabetes.co.uk/forum/attachments/insulin-profiles-pdf.21831.
- Kinnunen HM, Shamra V, Contreras-Rojas LR, et al. (2015). A novel in vitro method to model the fate of subcutaneously administered biopharmaceuticals and associated formulation components. Journal of Controlled Release 214: 94-102. doi:10.1016/j.jconrel.2015.07.016.
Due to the novelty of Sirius Scissor, Sirius Analytical wish to generate more data to further validate the instrument with different types of formulations (e.g. peptides, coagulation factors). Sirius Analytical are seeking partners with access to compound and formulation information (including existing preclinical/clinical data) that can be used in the instrument’s development.
In order for Sirius Analytical to expand the scope of their system, and assess the suitability of Sirius Scissor in predicting formulation effects following intravitreal administration, they would like to work with experts in this field.
Sirius Analytical are also interested to hear from potential users who may wish to implement this technology in their fundamental research and development programmes as they are keen to promote their technology to a wider user base. By having a lab-based instrument that can generate data within hours, the decision making process regarding the development of subcutaneous formulations is accelerated decreasing the time and costs of the project and avoiding testing formulations on animals which are not predictive of human responses.
Information about IP
No IP issues as all IP rights belong to Sirius Analytical. The IP that the project may generate will belong to Sirius Analytical.
Issues concerning the formulation of a potential new therapeutic can delay its development, contribute to the high attrition rates seen currently in drug development, and necessitate repetition of animal tests following optimisation of the formulation (Basavaraj and Betageri 2014). Sirius Scissor aims to overcome this by recreating biorelevant study conditions in vitro. The technology is able to act as an intermediate in the drug development process allowing organisations to produce many different formulations that can be quickly tested and adapted according to need. This ensures that formulations with poor performance or stability upon injection will be identified early in development so that they do not progress to animal studies. It is difficult to quantify specifically the level of reduction that may be possible as little information exists on the level of repetition of animal studies that occur due to formulation issues, however such repetition will be prevented by using Sirius Scissor resulting in a reduction in the number of animal studies carried out in the pharmaceutical and biotechnology industries.
Sirius Analytical have started with the subcutaneous route but plan to expand their portfolio to other delivery routes, therefore decreasing further the need for animal studies in the future.
Reference
- Basavaraj S, Betageri GV (2014). Can formulation and drug delivery reduce attrition during drug discovery and development—review of feasibility, benefits and challenges. Acta Pharmaceutica Sinica B 4 (1): 3-17. doi: 10.1016/j.apsb.2013.12.003