TruLarv Galleria mellonella - a model host for infection studies, toxicity testing and drug development (Partnering with Envigo)

At a glance
Contents
Overview
There is an increasing need for models that bridge the gap between in vitro studies and mammalian studies in order to improve the predictivity of preclinical testing and reduce late-stage attrition. Galleria mellonella larvae have been widely used over the past five years as non-mammalian models of microbial infection and for antimicrobial drug screening. More recently, the larvae have been used to test chemical toxicity and may be able to provide a more predictive screen prior to mammalian toxicity testing.
BioSystems Technology Ltd. has developed TruLarv; research grade Galleria mellonella larvae for use in chemical and drug development pipelines as early screens for assessing efficacy and toxicity. TruLarv are weight and age defined, surface decontaminated and bred without the use of antibiotics or other drugs and as a result they behave more consistently and reproducibly than larvae available from other sources.
With the support of funding from CRACK IT Solutions, the Exeter-based BioSystems Technology team is working with Envigo to compare acute oral toxicity testing of 25 compounds in G. mellonella with existing data from cell culture methods. The aim is to assess whether TruLarv provides a more accurate predictor of the mammalian LD50 than cell culture IC50 measurements.
If data obtained with TruLarv is more predictive of mammalian toxicity than existing cell culture methods, TruLarv toxicity testing will be offered as a service from Envigo, enabling wider uptake of the model by industry and reducing animal use by facilitating selection of only the most promising leads.
Full details about this CRACK IT Solution can be found on the CRACK IT website.
Impact
The purpose of this study was to investigate the suitability of TruLarv™ (larvae of the wax moth Galleria mellonella) as a model host for assessing acute toxicity of chemicals. 30 chemicals were assessed in the study, with results compared against IC50 values derived from two different in vitro cytotoxicity test methods (3T3 NRU and NK NRU assays, published in the OECD 129 guidance document) and in vivo acute oral LD50 values from rodents.
Of the 30 chemicals, it was possible to calculate the LD50 values for 19 of them. Due to solubility issues, LD50 values had to be estimated for eight chemicals. The remaining three chemicals could not be dosed in the TruLarvTM model due to lack of solubility in available solvents. Treatment was performed by subcutaneous microinjection of 10 µL test chemical into a defined injection site (front proleg). All treated and untreated control larvae were incubated at 20 - 25°C and scored for morbidity at 24 hours, 48 hours and 72 hours post challenge and LD50 (mg/kg) values in TruLarv™ calculated.
Categorising the hazard class of TruLarv™ LD50 values was based on the Globally Harmonized System (GHS) for classification and labelling of chemicals:
- Category 5: >2000 mg/kg, non-toxic
- Category 4: >300 - 2000 mg/kg, weakly toxic
- Category 3: >50 - 300mg/kg, moderate - very toxic
- Category 1-2: < 50 mg/kg, toxic
TruLarvTM accurately predicted 100% of the chemicals (nine out of nine) classified as non-toxic (category 5) based on published acute oral LD50 values. In comparison, cell-based cytotoxicity methods had only 44% concordance (four out of nine) with the acute oral toxicity method, with the remaining five chemicals being predicted as weakly toxic or very toxic (category 1 – 4). For one chemical (dimethylformamide) the IC50 values in the two in vitro tests were wildly different, with the 3T3 NRU assay classifying the chemical as category 1-2, and the NK NRU assay as category 5.
TruLarvTM correctly predicted 80% of the remaining 10 chemicals as being in category 1-4, as defined by their acute oral LD50 values. However it was less accurate at determining which specific category these chemicals fell in to. In comparison, cytotoxicity methods predicted 100% of chemicals as being classified in category 1 – 4, but again were limited in predicting a specific hazard category.
For full results, see Table 1 below.
The potential for current cell-based cytotoxicity assays to identify false positive results triggers further regulatory in vivo studies. The results obtained in this study give an indication that the TruLarvTM model is potentially superior to these in vitro methods for predicting low-toxicity chemicals. Addition of this model to European Chemicals Agency (ECHA) guidance as a recommended element in a weight of evidence approach could strengthen the options for registrants to justify the waiving of in vivo acute oral toxicity studies for chemicals predicted to be of low toxicity and lead to a reduction in numbers of animals used for toxicity testing.
Specifically, the 3Rs impact of a reliable ECHA approved non-animal technology (NAT) predictor of low chemical toxicity would potentially reduce the numbers of OECD 420 Acute Oral Toxicity studies required for chemical registration. OECD 420 requires a minimum of five animals per study. Approximately 150 OECD 420 studies were performed at Envigo in 2017 using approximately 900 animals. 95% of these animals had a mild severity rating indicating that in general the chemicals tested were of low toxicity. Based on the premise that TruLarv was part of an ECHA approved integrated WoE approach, it is estimated that the animal testing requirement could potentially be reduced by up to 50% (approximately 450 animals) following justified waiver of testing.
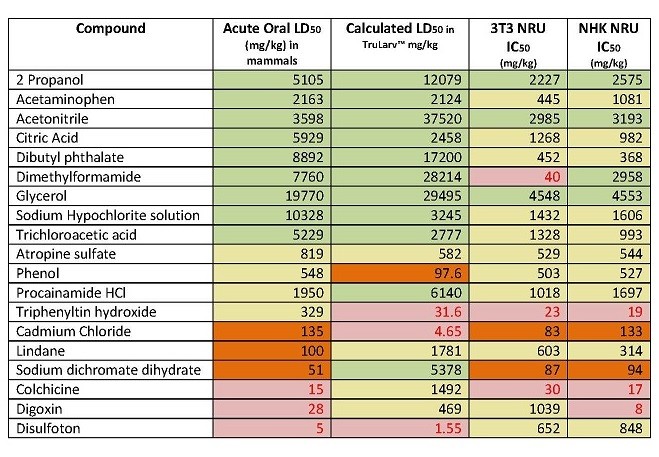

Table 1: Concordance of acute oral LD50 values categorised using the GHS with hazard categories predicted by TruLarv™ and cell-based cytotoxicity assays.
Publications
Allegra L et al. (2018). Galleria mellonella larvae allow the discrimination of toxic and non-toxic chemicals. Chemosphere 198:469-472. doi: 10.1016/j.chemosphere.2018.01.175.
