Microsampling
Guiding strategy and encouraging the adoption of microsampling techniques within any research or safety study.
On this page
Introduction
Advances in bioanalytical techniques have opened up the potential to use smaller sample volumes (microsamples) to assess drug and chemical exposure in blood, plasma and/or serum. It is now possible to assess drug levels and blood biochemistry parameters from samples of 5-20µl.
A microsample generally refers to a sample of ≤50µl. The small sample volume required enables samples to be taken from the main study animals which reduces or avoids the use of satellite animals. The sampling procedure provides a refinement because microsampling is quicker and less stressful, for example, warming of animals can be reduced or avoided.
Microsampling is used by many companies in early studies, such as those in discovery and for dose range finding. However there are scientific, business and animal welfare incentives to implement microsampling more widely within other toxicology and nonclinical studies where blood sampling is performed. Uptake for GLP toxicology studies was slower due to perceived regulatory hurdles and concerns that sampling from main study animals will compromise key endpoints. Many of these concerns have been addressed [1-4] and microsampling is now also routinely included in many regulatory studies. Additionally, updates to international regulatory guidance in 2017 [5] support and promote microsampling.
The aim of this web resource is to guide strategy and encourage adoption of microsampling techniques within academia and the pharmaceutical and chemical industries.
Benefits of microsampling
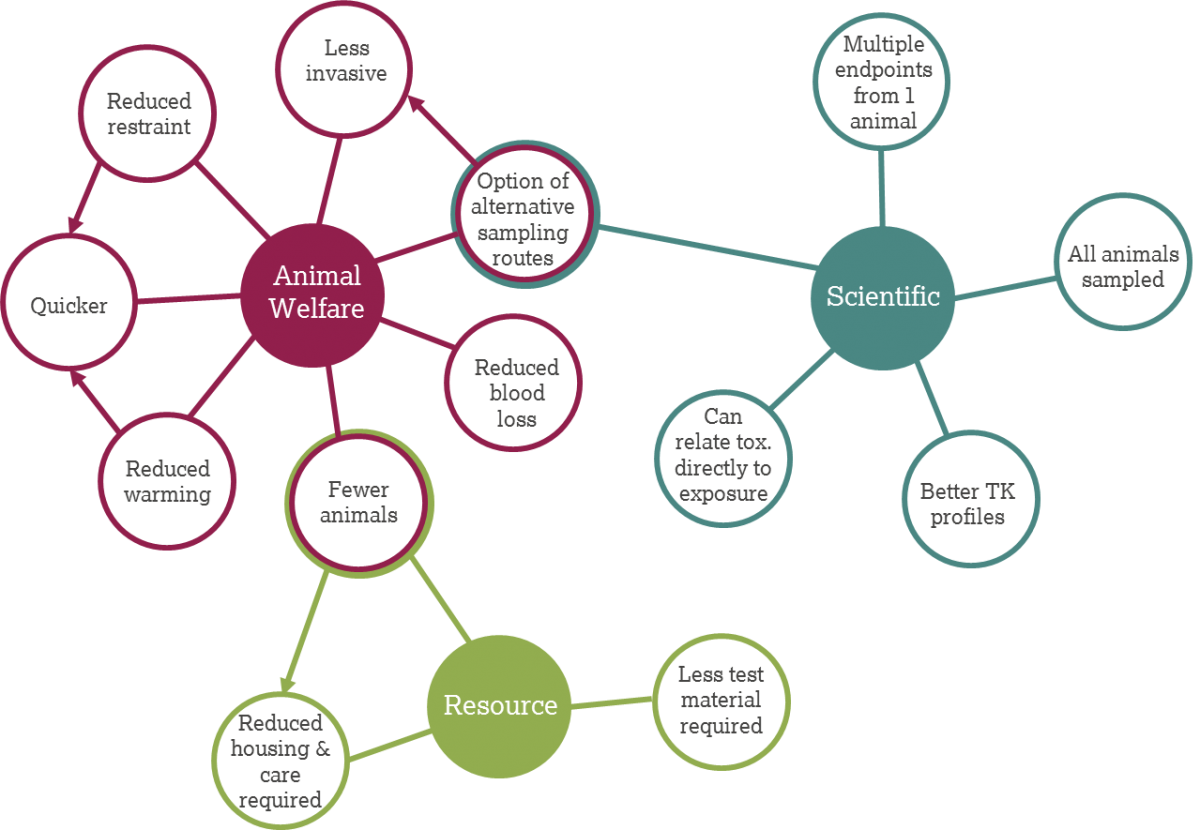
There are multiple advantages associated with microsampling including scientific, resource related and animal welfare benefits, which are summarised in the diagram above. Many aspects overlap (e.g., needing fewer animals leads to resource benefits of reduced housing and technician time).
Scientific benefits
- Ability to relate toxicity directly to exposure in the same animal
- All animals are sampled in the same way
- Multiple endpoints can be assessed in one animal
- More timepoints within toxicokinetic profiles
Animal welfare benefits
- Possibility of sampling from alternative routes
- Blood loss from the animal is reduced
- The procedure is less invasive
- Reduced restraint time or alternative restraining methods can be used
- Warming time is reduced
- Removing smaller volumes takes less time and is less stressful to animals
- No satellite animals or smaller satellite groups means fewer animals are required
Resource benefits
- Less test material is required
- Fewer animal-related resources, such as housing and care, is required
Can you use microsampling in your toxicology study?
There are many considerations that need to be taken into account to implement microsampling within your organisation and for a specific compound.
This decision tree can help to determine if your study is amenable to microsampling, and which is the most relevant approach (for example microsampling from a group of satellite animals or from main study animals).
For more information on sampling techniques in general, refer to our blood sampling pages.
Microsampling is applicable for any species; the advantages such as quicker sampling time and less-invasive procedure are refinements of equal benefit to non-rodents, where blood volume considerations are generally not as critical as for rodents.
Working group
To further increase uptake of microsampling, we lead an international microsampling user group which includes 27 companies and regulators. We act as an honest broker for data sharing - the evidence that we collated fed into the updates to regulatory guidelines [5] and is published within peer-reviewed journals [6-8].
Survey
We recently initiated another industry survey to establish how widely microsampling was used within the pharmaceutical and agrochemical industries. Data was collected between December 2020 and April 2021. Work is ongoing to address the current barriers identified.
Some of the results of the survey were presented at a talk by Dr Helen Prior, NC3Rs as part of our 2022 microsampling webinar.
Webinar (2022)
In September 2022, in collaboration with the British Toxicology Society (BTS), we hosted a webinar to raise awareness of the microsampling technique and encourage wider adoption across both discovery and regulatory toxicology studies. Recordings of the webinar presentations, including results from the 2021 survey, are available here [9].
Resources and references
- Powles-Glover N et al. (2014). Assessment of haematological and clinical pathology effects of blood microsampling in suckling and weaned juvenile rats. Regulatory Toxicology and Pharmacology 69(3):425-33. doi: 10.1016/j.yrtph.2014.05.00
- Powles-Glover N et al. (2014). Assessment of toxicological effects of blood microsampling in the vehicle dosed adult rat. Regulatory Toxicology and Pharmacology 68(3):325-31. doi: 10.1016/j.yrtph.2014.01.001
- Caron A et al. (2015). Clinical and anatomic pathology effects of serial blood sampling in rat toxicology studies, using conventional or microsampling methods. Regulatory Toxicology and Pharmacology 72(3):429-39. doi: 10.1016/j.yrtph.2015.05.022
- Hackett MJ et al. (2019). A Factorial Analysis of Drug and Bleeding Effects in Toxicokinetic Studies. Toxicological Sciences 170(1): 234-46. doi: 10.1093/toxsci/kfz092
- European Medicines Agency (2017). ICH Guideline S3A: Note for guidance on toxicokinetics: the assessment of systemic exposure in toxicity studies - questions and answers
- Sparrow S et al. (2011). Opportunities to minimise animal use in pharmaceutical regulatory general toxicology: A cross company review. Regulatory Toxicology and Pharmacology 61(2): 222-9. doi:10.1016/j.yrtph.2011.08.001
- Chapman K et al. (2014). Overcoming the barriers to the uptake of nonclinical microsampling in regulatory safety studies. Drug Discovery Today 19(5): 528-32. doi:10.1016/j.drudis.2014.01.002
- Chapman K et al. (2014). Reducing pre-clinical blood volumes for toxicokinetics: toxicologists, pathologists and bioanalysts unite. Bioanalysis 6(22): 2965-2968. doi: 10.4155/bio.14.204
- "Microsampling in toxicology: Maximising the scientific, business and 3Rs advantages". Webinar recordings.
A curated bibliography of microsampling papers from external sources is also available.
Other microsampling resources
Techniques for blood sampling in various laboratory animal species.