ZeGlobalTox is a medium/high-throughput preclinical zebrafish embryo screen capable of identifying compounds with potential cardiac, hepato- and neurotoxicity liabilities in the same animal. This has the potential to reduce the number of compounds entering regulatory in vivo studies and will reduce costly late stage attrition. ZeClinics seek collaborators that can provide small compound sets or expert guidance to help develop the platform and improve its utility for the end-user.
Through CRACK IT Solutions, ZeClinics collaborated with two companies, Noldus and Pivot Park Screening Centre. With the Solutions funding awarded the consortium validated the ZeGlobalTox platform against compound sets with known preclinical and clinical toxicity data.
There is increasing recognition that new approaches are needed to bridge the gap between preclinical in vitro and in vivo testing to reduce costly late-stage attrition of new drugs. Early in vitro preclinical studies, i.e. High Content Screening (HCS) of cell cultures, remain poor predictors of absorption, distribution, metabolism and excretion (ADME) of the drug of interest in a whole-organism. Subsequent in vivo studies, typically in rodents, help to understand the possible toxic properties of new drugs, but these are time consuming, expensive and use considerable numbers of animals.
Pharmaceutical companies and regulatory agencies are addressing this problem by searching for novel models and introducing new standards in drug development (Giovannetti et al., 2012). According to FDA recommendations, using zebrafish in the preclinical development of novel drugs could save $100 million before these drugs reach clinical phases (Food and Drug Administration report, 2014). High-throughput zebrafish screens are becoming an important tool in the pharmaceutical industry for assessing toxicity and efficacy of new drugs (Augustine-Rauch et al., 2010; Ali et al., 2011; Lieschke et al., 2007). At four days post fertilisation (dpf) the vital organs of zebrafish are already fully functional and any drug-induced effect can be easily detected and evaluated. Zebrafish share a high degree of conservation with humans in both their genome and their physiological processes. As recently shown toxicology studies performed in zebrafish embryos highly correlate with studies performed in rodents (Ali et al., 2011), making the zebrafish an excellent alternative model. Although single organ specific toxicity assays have already been commercialised, it is not yet possible to assess toxicity in the three main organs (brain, heart and liver) in the same animal.
References
- Ali S, van Mil HGJ, Richardson MK (2011). Large-Scale Assessment of the Zebrafish Embryo as a Possible Predictive Model in Toxicity Testing. PlosOne 6(6): e21076. doi:10.1371/journal.pone.0021076.
- Augustine-Rauch K, Zhang CX, Panzica-Kelly JM (2010). In vitro developmental toxicology assays: a review of the state of the science of rodent and zebrafish whole embryo culture and embryonic stem cell assays. Birth Defects Res 90(2): 87-98. doi:10.1002/bdrc.20175.
- Giovannetti G, Jaggi G, Ernst & Young’s Beyond borders Global biotechnology report 2012.
- Lieschke GL, Currie PD (2007). Animal model of human disease: zebrafish swim into view. Nature Rev Genetics 8(5): 353-67. doi:10.1038/nrg2091.
- U.S. Department of Health and Human Services, FDA report: “Innovation or stagnation: challenge and opportunity on the critical path to new medical products”.
ZeGlobalTox have created a new genetically modified line of zebrafish to drive the expression of unique fluorescent proteins in the three main organs (brain, heart and liver) enabling assessment of acute multi-organ toxicity and teratogenicity in the same animal. These modified Zebrafish embryos can be incubated with a drug and the functional changes imaged and analysed in an automated system composed of the following steps and parameters:
- Video-based tracking to detect and quantify locomotor activity, as a measure of brain functionality. The video below shows zebra fish being tracked in 96 well plate.
- Fluorescent microscopy scan of the heart, brain and liver to analyse morphological and structural alterations and teratogenic phenotypes, i.e. body deformity, oedema, tail detachment, pigmentation (Augustine-Rauch et al., 2010, Ali et al., 2011).
- Assessment of acute toxicity, i.e. LC50, NOEC, LOEC (Augustine-Rauch et al., 2010, Ali et al., 2011).
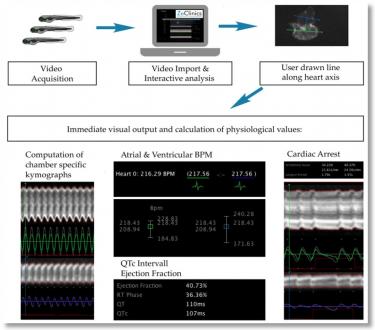
- Video recording of heart beat activity, with concurrent electrocardiogram, to detect any potential functionally altered activity such as heart frequency changes, QT prolongation and impaired Ejection Fraction (Wen et al., 2012). (Figure 1.)
- Liver dissection for molecular analysis of morphology and function, i.e. liver necrosis, heatomegaly, steatosis (Hill et al., 2012), by staining-based quantitative methods. This is currently done manually, but ZeClinics are exploring opportunities for automating the dissection.
The advantages of using zebrafish for drug screening are multiple:
- Drugs are administered directly in their tank water, saving time and helping to understand how molecules behave in terms of ADME.
- The small size and high number of zebrafish progeny allows parallel and reproducible testing of several drugs and doses in simple multiwell plate assays.
- Zebrafish embryos are optically transparent. Transparency, combined with fluorescent tissue-specific zebrafish transgenic lines, plus novel advances in imaging and processing, allows the in vivo effects of drugs in groups of cells or single tissues to be analysed.
ZeGlobalTox dramatically reduces time, costs and animal use in preclinical drug development. The entire process from treatment of the animals to image acquisition and analysis is automated, making the technology easy to adopt and integrate into existing testing strategies. The ZeGlobalTox technology could potentially be applied to a wider range of industries – biomedical research, cosmetics, consumer products, food, wastewater, chemical, and agriculture.
References
- Ali S, van Mil HGJ, Richardson MK (2011). Large-Scale Assessment of the Zebrafish Embryo as a Possible Predictive Model in Toxicity Testing. PlosOne 6(6): e21076. doi:10.1371/journal.pone.0021076.
- Augustine-Rauch K, Zhang CX, Panzica-Kelly JM (2010). In vitro developmental toxicology assays: a review of the state of the science of rodent and zebrafish whole embryo culture and embryonic stem cell assays. Birth Defects Res 90(2): 87-98. doi:10.1002/bdrc.20175.
- Hill A, Mesens N, Steemans M et al. (2012). Comparisons between in vitro whole cell imaging and in vivo zebrafish-based approaches for identifying potential human hepatotoxicants earlier in pharmaceutical development. Drug Metab Rev 44(1): 127-40. doi:10.3109/03602532.2011.645578.
- Wen D, Liu A, Chen F et al. (2012). Validation of visualized transgenic zebrafish as a high throughput model to assay Bradycardia related cardio toxicity risk candidates. Journal of App.Tox. 32(10): 834-42. doi:10.1002/jat.2755.
ZeGlobalTox are seeking collaborations with researchers or companies to help develop the platform to improve its utility for the end-user. ZeGlobalTox are specifically looking for partners able to:
- Provide small compound sets with known preclinical and clinical (if available) toxicity profiles with which to validate the ZeGlobalTox platform.
- Provide advice and guidance on experimental approaches for single organ toxicity assays and how these could be integrated into the triple transgenic zebrafish line.
- Help automate the platform and develop software for image and behavioural analysis.
ZeGlobalTox uses zebrafish at life stages that fall outside of the regulatory framework, ie before five dpf, replacing the need to use protected mammals. Zebrafish embryos and larvae allow for rapid and detailed phenotypical screening of the major organs affected by drug toxic effects and can bridge the gap between preclinical in vitro and in vivo screens, enabling ADME properties to be assessed in a whole organism. This has the potential to reduce the number of compounds entering in vivo studies which are likely to fail because of poor ADME that has not been picked up in earlier studies.
ZeGlobalTox assesses cardiac, brain and liver toxicity in the same animal, potentially replacing by up to a third the number of traditional rodent studies required to assess toxicity in these organ systems. Traditional in vivo testing strategies require up to 20 rodents per organ assay (60 animals for cardiac, brain and liver toxicity testing). Using ZeGlobalTox, only 20 non-protected animals are required to generate the same data.
Additionally, the size and transparency of zebrafish larvae enables minimally invasive organ-specific assays, including microscopy, to be performed. This negates the need for more invasive assays in mammals to assess arrhythmias, QT prolongation, and mechanical myocardial dysfunctions, for example.
Overview | Impact | Publications
Overview
To maximise the potential 3Rs and commercial benefits of ZeGlobalTox, the team will validate the platform against compound sets with known preclinical and clinical toxicity data and are seeking to automate the complete procedure. Through the CRACK IT Solutions scheme, they are collaborating with two companies, Noldus and Pivot Park Screening Centre to adapt their advanced imaging and behavioural analysis software and automated robotic platform for a better end-user uptake.
Impact
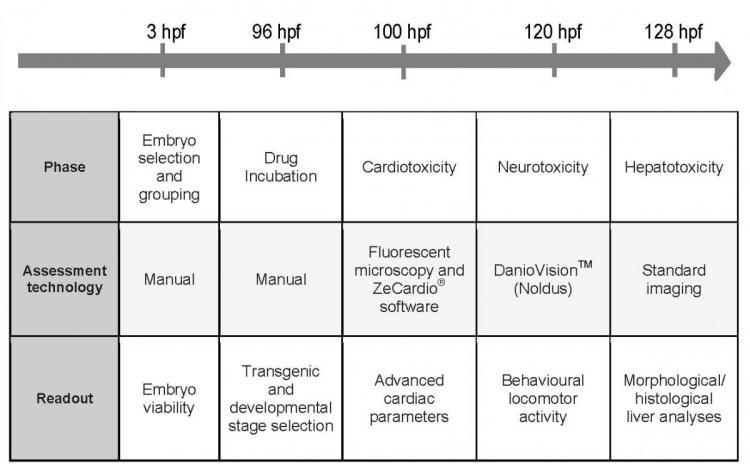
To assess ZeGlobalTox validity, it was first necessary to optimise the experimental conditions for each of the single-organ toxicity assays. The team at ZeClinics S.L. used gold-standard drug controls to examine cardiovascular function, locomotor activity and liver function. This phase also provided an important opportunity to assess the utility of the software and imaging technologies developed or adopted to examine each of the specific endpoints within the combined assay. The fully optimised combined protocol (see Table 1) was validated with a set of four positive compounds and an additional group of 20 blind compounds, provided by the collaborator Pivot Park Screening Centre.
Table 1: ZeGlobalTox final procedure.
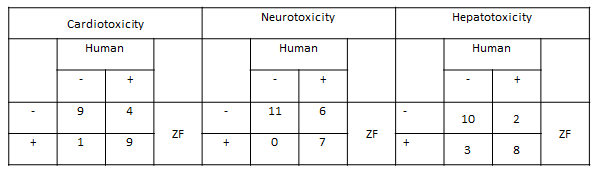
Each tested drug has been correlated to its known effects in humans, as identified in the scientific literature, and compared with the phenotype measured in the ZeGlobalTox assay. Using this analysis, it is possible to determine, by multiple parameters, the predictability of the ZeGlobalTox approach on the three main organ-specific drug toxicities from the same zebrafish larva. It is important to note that cardiotoxicity was segmented in four different phenotypes (QTc prolongation, cardiac arrest, frequency and ejection fraction), which gives this assay further relevance. Full results are shown in Tables 2 and 3.
Table 2: ZeGlobalTox results for 23 compounds assessed for cardiotoxicity and hepatotoxicity and 24 compounds for neurotoxicity. Cardiotoxicity prediction has been segmented in four different phenotypes (96 predictors vs 23). ZF: zebrafish.
| Assay | Specificity | Sensitivity | Accuracy | PPV | NPV |
| Cardiotoxicity | 0.90 | 0.69 | 0.78 | 0.90 | 0.69 |
| Neurotoxicity | 1.00 | 0.54 | 0.75 | 1.00 | 0.65 |
| Hepatotoxicity | 0.77 | 0.80 | 0.78 | 0.73 | 0.83 |
| ZeGlobalTox | 0.89 | 0.68 | 0.77 | 0.88 | 0.72 |
Table 3: ZeGlobalTox Predictivity. Specificity (TN/(TN+FP), Sensitivity TP/(TP+FN), Accuracy (TP+TN)/(TP+TN+FP+FN); PPV: TP/(TP+FP); NPV: TN/(TN+FN). PPV: positive predictive value, NPV: negative predictive value, TN: true negative, TP: true positive, FN: false negative, FP: false positive.
This validation project highlights the predictive value of the combined ZeGlobalTox assay when assessing a relatively small compound set – see here for the full compound set. Further exploitation of ZeGlobalTox could have a large impact on the number of animals used in toxicity studies. A traditional in vivo single-organ test is conducted, on average, with a group of 20 rodents or dogs per tested drug, resulting in up to 60 animals being used to test neuro-, cardiac- and hepatoxicity of each drug. Using ZeGlobalTox, it is possible to reduce this number to a third, plus zebrafish is a non-protected animal when used under 5 dpf (EU Directive 2010/63/EU). The optimization of multiple physiological parameters included in ZeGlobalTox, either by using ZeCardio® software (also developed as a result of a collaboration identified through CRACK IT Solutions – see here) or adapting already available technologies, such as DanioVisionTM, from Noldus, has contributed to improving the utility and usability of ZeGlobalTox, making it easier for wider adoption of end-users.
The first version of the ZeGlobalTox technologies has been commercialised and is already being used by Dompé Farmaceutici S.p.A. and a research group at Qatar University, to better characterise the toxicological profile of their compounds. This demonstrates the interest of both academia and private industries, key market segments for ZeClinics. Moreover, the company is in advanced negotiations with several other potential clients.
ZeClinics is continuing to develop and expand the technology by exploring further options for automation to improve throughput and exploring the methodology as a tool for efficacy studies in the fields of neuro- and cardioprotection. This includes the introduction of more complex behavioural phenotypes and in-house drug discovery projects to identify natural substances with protective effects.
Importantly, the initial work done under the CRACK IT program, has helped ZeClinics to raise several other public non-dilutive grants, including the most ambitious European grant for SMEs, the SME Instrument Phase 2, with €1.87m for developing a fully-automated version of the ZeCardio high-throughput cardiotoxicity screen.
Publications and presentations
Publication
- Cornet C, Calzolari S, Miñana-Prieto R, et al. (2017). ZeGlobalTox: An Innovative Approach to Address Organ Drug Toxicity Using Zebrafish. Int. J. Mol. Sci. 18(4): 864; doi:10.3390/ijms18040864.
Poster
- Cornet C et al. (Eurotox, 2016). ZeGlobalTox: An Innovative Approach to Address Organ Drug Toxicity Using Zebrafish.
- Cornet C et al. (10th European Zebrafish meeting, 2017). ZeGlobalTox: An Innovative Approach to Address Organ Drug Toxicity Using Zebrafish.
Oral Presentations
- ZeGlobalTox - An Innovative Approach to Address Organ Drug Toxicity Using Zebrafish. Davide D'Amico, Ph.D., CEO, Management Board, ZeClinics. World Preclinical Congress Europe, November 16th 2017.