Research round-up: 3Rs innovations in Alzheimer's disease
Find out about the £3M the NC3Rs has invested to replace, reduce or refine the use of animals in research to better understand and treat Alzheimer’s disease.
Contents
Introduction
Alzheimer’s disease is responsible for 60 to 70% of the 55 million cases of dementia worldwide. Treatments are limited to symptom management and there are no drugs available to slow, or reverse, the death of brain cells and disease progression. Typically, research into Alzheimer’s disease and other diseases of the brain has relied on animal experiments, usually using rodents, to understand how neurons talk to each other and how the brain functions. Alzheimer’s disease research often involves mice carrying genetic alterations that cause specific proteins, called amyloid-β and tau, to build-up in the brain. Accumulation of these proteins drives Alzheimer’s disease in human patients, so scientists can use these genetically altered mice to mimic some aspects of Alzheimer’s disease and investigate changes in the mouse’ brain and behaviour.
The mice are used in a range of different experiments. This includes behavioural experiments to assess changes in memory and decision-making, or implanting probes in the brain to measure the electrical activity of neurons. The accumulation of amyloid-β and tau proteins in the brains of mice can lead to severe atrophy (shrinkage) of the brain. Some of the tests involved can cause pain, suffering or distress for the animals involved and humane endpoints where the mouse is killed to prevent unnecessary suffering, are used. After culling the animals, scientists will often study the mouse’ brain to look for physical and cellular changes associated with Alzheimer’s disease.
We are driving innovative approaches to replace, reduce and refine the use of mice in Alzheimer’s disease research. Since our first project in the field in 2009, we have invested £3M to embed the 3Rs in research to better understand and treat Alzheimer’s disease.
Replacing mice with human mini-brains
The lack of effective drugs to treat Alzheimer’s disease suggests there are important differences between mouse models of the disease and what happens in human patients. Replacing the use of mice with human cells aims to improve the translation of preclinical research into improvements in treatment.
Professor Selina Wray at University College London (UCL) is tackling this translational problem using miniature brain ‘organoids’ grown from patient cells. Selina created these mini-brains to solve the UnTangle CRACK IT Challenge, funded by the NC3Rs and Alzheimer’s Research UK, and additionally sponsored by pharmaceutical companies Lilly and Janssen. We funded Selina to create a human-relevant assay to test new drugs targeting tau protein in Alzheimer’s disease. Selina’s 3D brain organoids show accumulation of tau protein and can replace the use of mice for some studies.
Alzheimer’s disease can cause changes in the brain before clinical symptoms develop, making early diagnosis and treatment difficult. We are now funding Dr Marc Busche and PhD student Francesca Lam to collaborate with Selina, combining her organoid technology with Marc’s expertise in high-resolution imaging. Marc and Francesca are studying circuits of neuronal cells in the 3D brain organoids and aim to demonstrate that organoids can replace animal models in experiments to understand the early changes in cell signalling which underpin Alzheimer’s disease. Ultimately, they aim to use the organoids to identify early biomarkers of Alzheimer’s disease and develop treatments for asymptomatic patients.
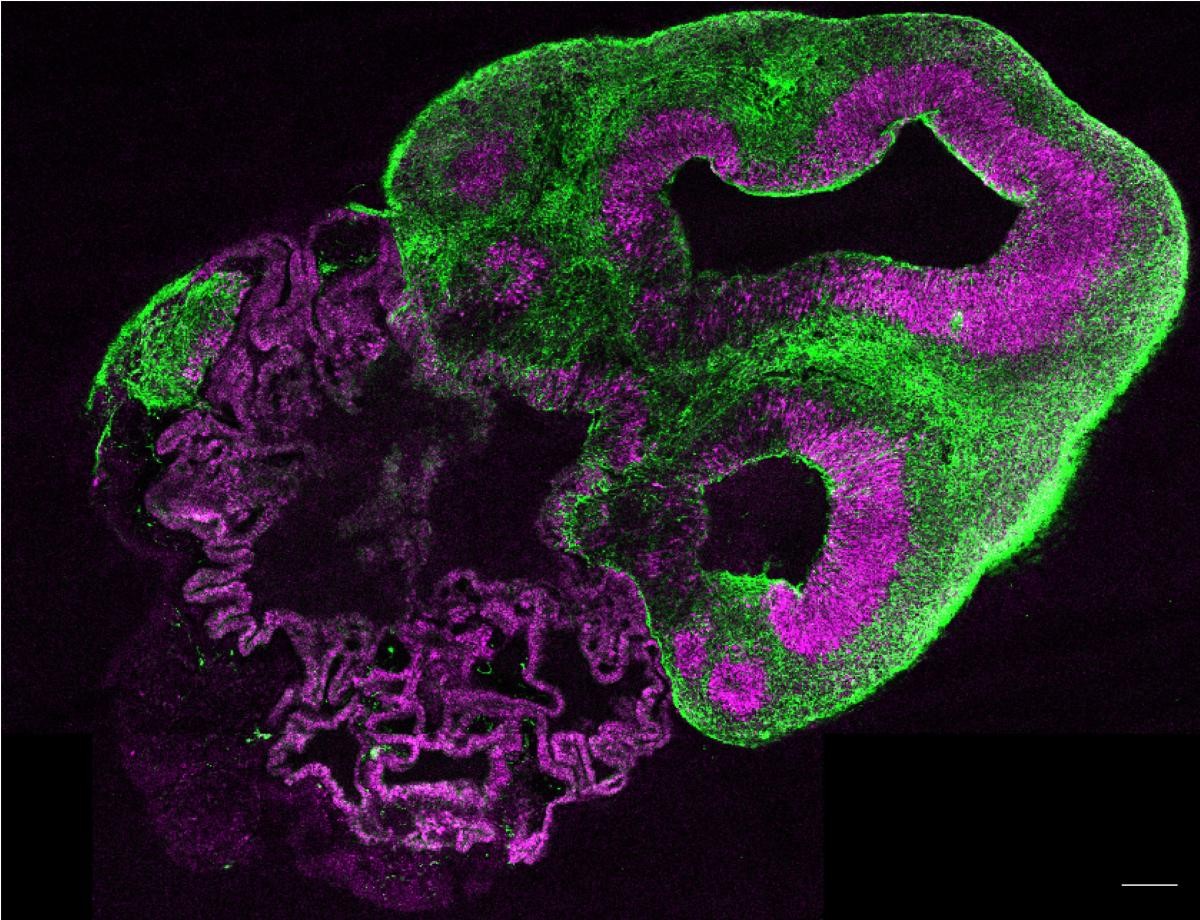
The very first brain organoids were created by Dr Madeline Lancaster, who won the International 3Rs Prize for the work in 2016. The Prize, awarded by the NC3Rs and sponsored by GSK, supported Madeline to purchase new equipment for her lab at the MRC’s Laboratory of Molecular Biology, where she is now studying the cellular mechanisms that drive degenerative diseases of the brain and aims to develop new therapeutic options. We awarded Professor Simon Schultz a Skills and Knowledge Transfer grant to transfer Selina and Madeline’s brain organoids to his lab at Imperial College London so that he can investigate their suitability for replacing mouse use in his research on neural circuits, which are altered in Alzheimer’s disease. Simon is demonstrating the utility of brain organoids for neuroimaging studies, where scientists visualise the activity of cells in the brain, which are typically done in mice and require surgery and other invasive procedures.
Minimising the use of mice
When tau builds up in the brain, the protein becomes misfolded and formed tangles in neurons (called neurofibrillary tangles, NFTs). These NFTs contribute to neurodegeneration but it remains unclear exactly how tau causes brain cells to die. One problem faced by scientists is that it is very difficult to collect and study dying cells. When Professor Maria Grazia Spillantini and Dr Aviva Tolkovsky at the University of Cambridge were awarded their NC3Rs Project grant in 2014, the only cellular models of Alzheimer’s disease did not form NFTs or lead to neuronal cell death, meaning scientists were wholly reliant on animal models to study these processes. Maria and Aviva were able to isolate and grow dorsal root ganglia (DRGs) from mice with a human mutant tau protein. DRGs are a type of neuron that form a bridge between the peripheral and central nervous systems. By growing DRGs in the lab at different stages of tau build-up and NFT formation, the cellular processes which lead to cell death in Alzheimer’s disease can be studied whilst simultaneously reducing the number of mice needed for these experiments.
Cell-based models may not reflect some of the more complex cellular or physiological features of the brain, including the tau protein tangles and amyloid-β plaques seen in Alzheimer’s disease. For this reason, scientists studying the brain may use slices of brain tissue which are collected from animals killed specifically for this purpose. We funded Dr Wendy Noble and Dr Diane Hanger at King’s College London to develop a new method of generating brain tissue slices and reduce the number of mice needed. Using their method, during her PhD research Dr Cara Croft demonstrated that it was possible to collect up to 36 slices from an individual mouse brain and grow them in the lab for up to six months. This means the slices can be used to follow biochemical and physiological changes associated with Alzheimer’s disease progression over time and avoids the need to collect brain tissue from multiple mice at different ages.
Hear more from Wendy and Cara about publishing this research in the NC3Rs F1000 open access gateway – First out of the gate(way): a method for studying Alzheimer’s in vitro.
Realising the 3Rs potential of flies
Fruit flies (also known by their Latin name, Drosophila) are used extensively in biological research to address a range of scientific questions from physiology to neuroscience. According to current scientific thinking, they are unable to experience pain, suffering or distress. This means that they can be used as a so-called partial replacement to replace mice and other vertebrates in research, providing an integrated system to study biology but without the concerns associated with using other animals. However, further development and demonstration of their utility is needed to build confidence and awareness across the scientific community. We fund a range of projects across multiple fields of research to realise the replacement potential of fruit flies.
Our first Alzheimer’s disease replacement project came from Professors Kevin Moffat and Bruno Frenguelli at the University of Warwick in 2010, who developed the Drosophila fruit fly as an alternative to rodent models of Alzheimer's disease. Critically, they showed for the first time that a human enzyme interacts with tau and promotes neurodegeneration in the fly. Fruit flies have become an established and effective model of Alzheimer’s disease as they respond to overexpression of amyloid-β and tau proteins in similar ways to humans, resulting in neuronal cell death and symptoms such as reduced movement.
Dr Frances Wiseman and Dr Teresa Niccolli are collaborating at UCL to demonstrate the utility of flies to study early onset Alzheimer’s disease in people with Down syndrome (AD-DS). By the age of 40 nearly all people with Down syndrome develop amyloid-β plaques and tau tangles, leading to AD-DS before the age of 60 in most individuals. One of the genes on chromosome 21 (which people with Down syndrome have an extra third copy of) forms amyloid-β, partially explaining their increased risk of early onset Alzheimer’s disease. But, in previous experiments using mice, Frances has shown that other genes may be involved. We funded Frances to work with PhD student Katharina Wenz to replace their mouse model with Drosophila which model both Alzheimer’s disease and Down syndrome, to further investigate which other genes on chromosome 21 are responsible for driving AD-DS.
During his first NC3Rs grant, Dr Alessio Vagnoni at Kings College London developed a technique to replace some vertebrates in ageing research, by non-invasively visualising the transport of molecules along neurons in the transparent fly wing. Alessio was awarded an NC3Rs Skills and Knowledge Transfer grant to transfer this technique to Dr Tito Cali’s lab at the University of Padova in Italy, and replace the use of mice and zebrafish in Tito’s research on organelle function and interaction in neuronal ageing. Organelles, such as mitochondria and endoplasmic reticulum (ER), are complexes in cells that are critical for cellular function. Experiments using the replacement Drosophila model have shown that reduced movement of mitochondria, and fewer interactions between mitochondria and ER, contribute to ageing in neurons. In his new NC3Rs Studentship Alessio will apply the Drosophila model to study neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis, demonstrating the potential to replace mice and zebrafish in further areas of ageing research.
Harnessing technology in mouse behavioural experiments
There are more alternatives than ever before for scientists to investigate the cellular and physiological basis of Alzheimer’s disease without the use of vertebrates such as mice. Where they are still required, for example in experiments to assess changes in cognition, behaviour and memory, we aim to reduce the numbers of animals needed by supporting good experimental design and refine experiments to maximise animal welfare.
Dr Alex Easton was able to halve the number of rodents needed for spontaneous recognition tasks in his research at Durham University. These tests are used to assess memory loss and decline in cognitive function in Alzheimer’s disease and other models of neurodegeneration and ageing. They involve the animals exploring objects in their environment – rodents prefer exploring novel objects and this means that scientists can assess whether they remember any of the items. Alex developed a ‘continuous trial apparatus’ which allows rodents to explore multiple items in a single session, rather than one trial per animal, or one trial per day. The traditional single trial set up required 16 to 20 mice being tested repeatedly for multiple days, whereas Alex’s apparatus generates highly reliable data using just eight mice with fewer sessions. This means shorter trial times and avoids additional handling of the animals between trials, reducing their stress levels and allowing scientists to better assess behaviours relevant to memory and cognition.

The multi-trial system to reduce the use of rodents in memory research was developed by Alex and Kamar Ameen-Ali in an NC3Rs-funded Studentship. Kamar developed a protocol that harmonises memory tests used in rodents and humans and showed a direct link between cognitive processes in rat and human test subjects. This means Kamar and Alex could test recollection across species using the same experimental approach, improving the translation of animal behavioural experiments to patients. Whilst originally designed for rats, Alex has since collaborated with GSK to adapt the apparatus for mice and has developed a semi-automated version including a camera to monitor behaviour and an app for behavioural scoring. Working with GSK Alex has been exploring ways to maximise the usability of the apparatus for industry, and collaborating with other institutions and research groups, to further reduce the use of rodents in memory research.
Find out more about Kamar's research: Improving the translation of memory research from animals to humans.
To understand the neural processes that underpin behaviour, and how these change in disease, scientists record the activity of neurons in the mouse brain. This has typically required surgically implanted probes with physical wires, which impede the mouse’ movement, limiting the amount of useful data scientists can collect and affecting animal welfare. Wireless recording technology allows greater freedom of movement, but until recently most devices weighed upwards of 4g – equivalent to a mouse carrying 10% of their bodyweight on their head. Professor Esther Rodriguez-Villegas at Imperial College London solved the Cognition CRACK IT Challenge that aimed to address this, sponsored by Lilly. With NC3Rs funding, she developed an ultralight weight wireless device for recording neural activity from the brains of mice. Called TaiNi, the devices’ small size and light weight (just 1.5g) allows scientists to record information as the mouse performs a full range of its normal behaviours. We supported the transfer of the TaiNi system from co-developer Lilly to the research groups of Dr Jonathan Brown and Dr Michael Craig at the University of Exeter. Jonathan used the TaiNi system to improve the welfare of the mice used in his research into areas of the brain involved in memory and neural plasticity impaired in Alzheimer’s disease. You can see the difference in the activity of a mouse wearing a TaiNi device, compared to a tethered device, in the videos below:
Videos from: Zhou Jiang et al (2017) TaNi: Maximizing research output whilst improving animals' welfare in neurophysiology experiments. Scientific Reports doi: 10.1038/s41598-017-08078-8
Read more about the TaiNi device: New device for refined neural recording in mice could transform dementia research.
Discover NC3Rs-funded science to replace animals in research to understand and treat stroke.

Find out about the work we have funded to build the evidence base for refining the use of fish in research.

