Research round-up: Refining the use of fish in research

Find out about the work we have funded to build the evidence base for refining the use of fish in research.
Contents
Introduction
In 2022, 14% of regulated procedures on animals in the UK involved fish. The zebrafish (Danio rerio) is the most used aquatic species. Zebrafish and their embryos are used extensively for developmental and toxicology studies and are increasingly being used as alternatives to mammals to model biological and disease processes:
- The zebrafish genome is well defined and scientists can introduce genetic changes to mimic aspects of human disease.
- The larvae develop very quickly and biological processes can be studied over a few days, where similar experiments using mammals could last weeks or months.
- Zebrafish embryos are transparent and can be non-invasively imaged. They can be used as a partial replacement* for some in vivo experiments and in high throughput experiments, for example drug screening.
With the use of zebrafish increasing across research, ensuring they are reared with the highest welfare standards and experience as little pain, suffering and distress as possible is a priority. Refinements are important for both animals and science, as healthy animals that can display a full range of their natural behaviours produce scientific data that is more reproducible, reliable and relevant. We are continuing to fund research to refine the use of fish and produce guidance to help scientists and animal technicians make best practice common practice in their facilities and institutions.

Assessing pain and welfare
The ability to evaluate pain in animals is essential for their welfare so that scientists and animal technicians can identify and alleviate suffering, for example by administering pain relief. Monitoring the behaviour and welfare of fish can be challenging as they are housed in groups within tanks. Over a decade ago, we funded Professor Catherina Becker at the University of Edinburgh to build the evidence base for non-invasive measures of pain and distress in fish. Indicators of stress in fish include erratic movement, spending time at the bottom of the tank and high levels of the stress hormone (cortisol) in tank water. Catherina linked these non-invasive welfare markers to the molecular and cell signalling changes that occur in response to pain and went on to study how these are affected by pain-relieving drugs to identify effective analgesics for fish.
Earlier this year Dr Lynne Sneddon won the Charles River Laboratories Excellence in Refinement award for her expertise and dedication to improving laboratory fish welfare. We first worked with Lynne in 2012 when we awarded her a Project grant to non-invasively detect and assess pain in zebrafish. In her NC3Rs Project grant, Lynne developed the Fish Behaviour Index (FBI), an automated monitoring tool to detect subtle changes in behaviour of individual fish and support quicker welfare assessment, interventions and administration of analgesia. Using the FBI, Lynne built the evidence base for group housing of zebrafish demonstrating that socially housed fish had lower levels of stress and anxiety. The social interactions between fish within a tank, and the group behaviour of the shoal, are important markers of their welfare. In another NC3Rs Project grant, Professor Oliver Burman at the University of Lincoln used fish social networks to identify early behavioural changes that indicate stress and intervene sooner to improve welfare.

Read more about the Fish Behavioural Index in the news item: A new automated zebrafish behaviour monitoring tool.
Through her NC3Rs-funded work, Lynne also validated measuring cortisol levels in the water as a refined non-invasive method to assess stress, instead of culling individual fish to measure whole body cortisol. Dr Ioanna Katsiadaki further developed tank water sampling as a refined approach to assess fish welfare in her NC3Rs Project grant. Her work at the Centre for Environment Fisheries and Aquaculture Science analysed tank water for brain monoamines secreted by fish – these are neurotransmitters that regulate emotion, such as dopamine or serotonin. She investigated two different approaches to collect water samples and analyse water chemistry, aiming to develop a tool that can be used routinely across aquaculture to track welfare markers in tank water.
Find out more and hear Ioanna speak about her research in this blog article: Testing chemicals for endocrine disruption using fewer fish.
Building the evidence base for best practice
Many of the refinement projects we fund involve building a robust scientific evidence base to show that the new technique meaningfully improves animal health and welfare. This is important to make sure our guidance and recommendations support the highest animal welfare standards based on current scientific thinking. It also drives the uptake of refined methods by communicating the benefits for science, animals and those who work with them.
We are funding Professor Roderic Wilson at the University of Exeter to determine ideal ranges of major water parameters for zebrafish to build the evidence base for guidance and best practice in aquaculture facilities. For fish, water chemistry has profound impacts on their welfare, behaviour and physiology. For example, fish take up sodium and chloride ions from water to regulate the pH of their blood whilst calcium ions control gill and skin permeability. Even small increases in water CO2 levels can impair behavioural responses, learning and immunity in fish. Despite the importance of water chemistry for fish health and welfare, there is significant variation across aquaculture facilities and no ‘gold standard’ guidelines.
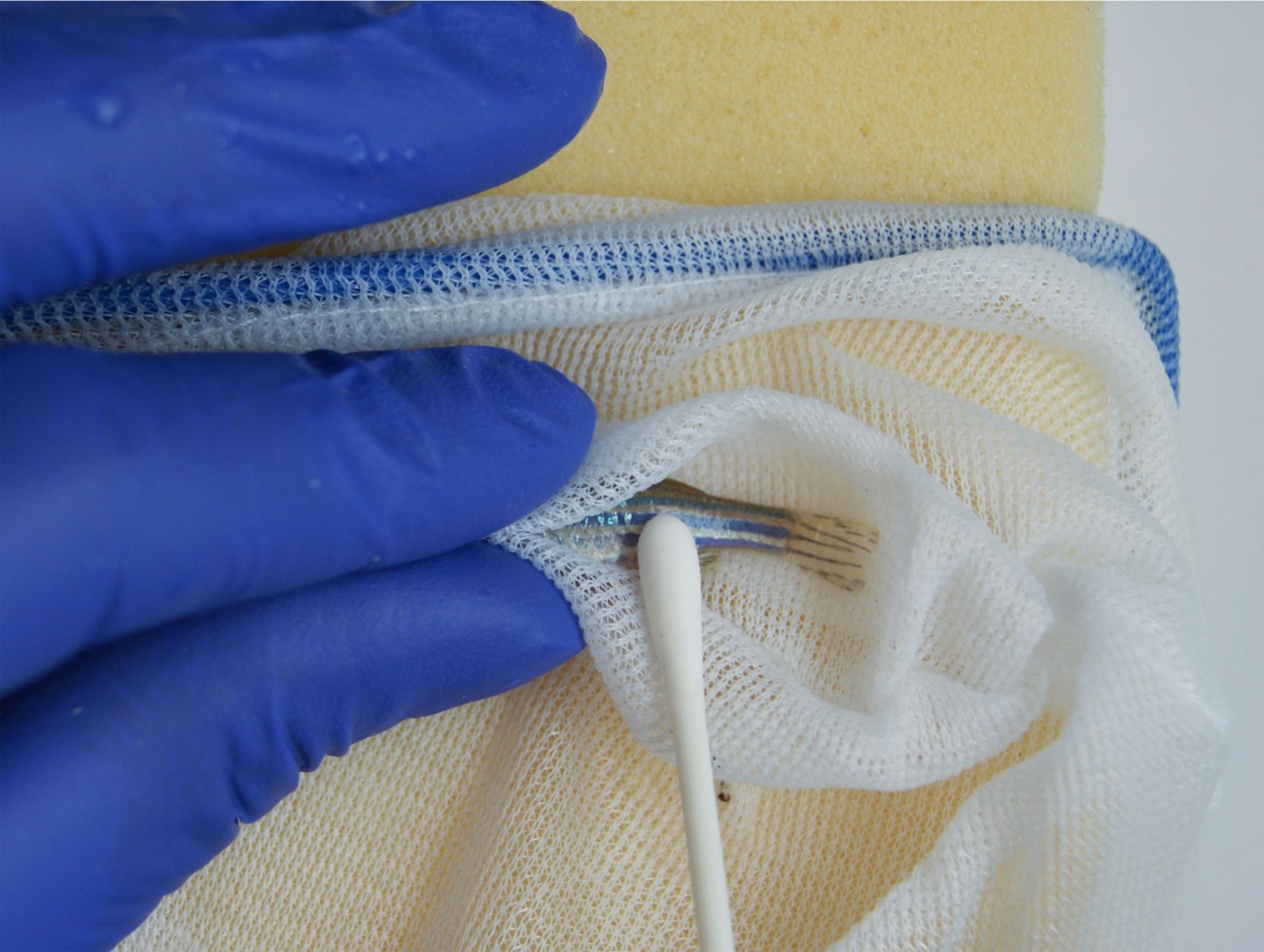
One of the most common procedures performed on laboratory zebrafish is fin clipping, with approximately 85% of the 3,250 zebrafish facilities worldwide using the technique. This involves cutting a section of fin tissue for DNA analysis. Fin clipping is typically performed under anaesthesia which has been shown to negatively affect fish welfare and the procedure can cause pain and stress and affect movement and behaviour. We funded Dr Will Norton at the University of Leicester to investigate a non-surgical alternative to refine the collection of DNA samples, called skin swabbing. Skin mucus samples can be collected by gently stroking the fish with a swab. The efficiency of skin swabbing for collecting sufficient DNA, faster and easier than by fin clipping was well understood, but there had been no systematic investigation of its impact on fish welfare. Will’s work showed that skin swabbing scored better than fin clipping for all measures of fish health and welfare and could have a significant welfare impact in fish research. We worked with the team to develop guidance resources to drive uptake of skin swabbing across animal research facilities.
From laboratory zebrafish to larger free living fishes and sharks, Dr Matthew Witt and PhD student Ghalia Abel at the University of Exeter are investigating refined procedures for electronic tagging used to study behaviour and ecology for conservation research. Tagging is an invasive procedure that requires boarding the animal on the boat, during which time their blood oxygen levels decrease. This stress affects the way that animals behave when they are returned to the water, with fish often swimming faster and in erratic directions immediately after release. However, there is currently little scientific data on the welfare implications of tagging wild fish. In her NC3Rs funded PhD, Ghalia is building the evidence base for refinements including the use of anaesthesia and flowing water over the gills to maintain respiration. Refining tagging procedures will minimise the stress experienced by wild fish and so reduce the variability of their behaviour. This will allow conservation scientists to collect more reliable data that better reflects the natural behaviour of fish.

Innovative assays
As well as refining the care, handling and husbandry of animals, scientists can apply advances in technology to minimise the impact of their experiments on fish welfare.
In an NC3Rs PhD Studentship, Dr Ralf Wenz in Professor Margaret Dallman’s lab at Imperial College London developed non-invasive techniques using fluorescent zebrafish and live imaging to investigate stem cell transplants, as an alternative to experiments that used fish survival as a measure of transplant success. We also supported work in the Dallman group using their advanced imaging techniques to study inflammation in response to high cholesterol diets and asthma in zebrafish models which replace the use of mammals.
PhD student Lisa King-Wolf is working with Dr Hanna Hartikainen at the University of Nottingham to refine the use of fish in proliferative kidney disease (PKD) monitoring. PKD is a severe infectious disease of salmonids, a family of fish including salmon and trout, for which there is no vaccine. Hanna’s Studentship aims to develop a monitoring toolkit for PKD, including a blood test to determine exposure and track the development of immunity. As well as avoiding the lethal sampling which is currently used this refined approach is more sensitive and informative, helping them to understand natural protective immunity and perform vaccine trials in fish populations.
- You can find out more about all the work we fund involving fish in our research portfolio.
- Guidance, information and recommendations for those working with zebrafish can be found on our zebrafish welfare guidance page.
* Partial replacement includes the use of some animals that, based on current scientific thinking, are not considered capable of experiencing suffering. This includes invertebrates such as Drosophila, nematode worms and social amoebae, and immature forms of vertebrates such as fish. The Home Office issues licenses under the Animals (Scientific Procedures) Act 1986 (ASPA) for the use of protected animals in scientific research and testing in the UK. Under the Act, a ‘protected animal’ is 'any living vertebrate, other than man, and any living cephalopod'. Larval forms of fish and amphibians are protected animals under ASPA once they are capable of feeding independently. For zebrafish, this is five days after fertilisation. More information on the definition of the 3Rs can be found in our What we do pages.
Discover NC3Rs-funded science to replace animals in research to understand and treat stroke.

Find out about the £3M the NC3Rs has invested to replace, reduce or refine the use of animals in research to better understand and treat Alzheimer’s disease.