Highlights from the 2019 NC3Rs/IAT Animal Technicians’ Symposium
In this blog post we look back at our 2019 Animal Technicians’ Symposium, a joint event with the Institute of Animal Technology (IAT).
As well as sharing the winners of the technicians' poster prize, we summarise key points from three of the day's talks, delivered by experts in laboratory animal welfare and refinement.
Do zebrafish have a taste for interior design? Can you train a laboratory mouse to be fearless? And do rats really enjoy being tickled? These were some of the topics discussed at the 2019 Animal Technicians’ Symposium, held in October and hosted by the NC3Rs in collaboration with the IAT. Over 150 technicians from more than 40 organisations in the UK and abroad registered for this year’s event, which featured talks on the latest refinement opportunities in animal research. Speakers included Dr Lynne Sneddon of the University of Liverpool, Ms Marie Eriksson from Research Institutes of Sweden (RISE) and Ms Megan LaFollette of Purdue University, USA, who all shared their expertise on how to improve laboratory animal welfare.
This year’s Symposium also celebrated the fundamental role animal care staff play in the refinement of animal procedures and husbandry, as well as their commitment to serving the 3Rs through their everyday work. Attendees were given the opportunity to showcase their innovative 3Rs ideas during a flash poster session, with three posters being awarded prizes.
- Improving zebrafish welfare through enrichment, behavioural assessment and analgesia
- Training techniques for less stressed laboratory rodents
- Tickling rats for better welfare
- Poster session prize winners
Improving zebrafish welfare through enrichment, behavioural assessment and analgesia
If you have worked with laboratory mice, rats or other mammals, you will know that environmental enrichment is important for their welfare, allowing them to satisfy their innate curiosity and perform natural behaviours. What about fish though? Is enrichment important for zebrafish too? And if so, what kind of enrichment do zebrafish want? According to Dr Lynne Sneddon, environmental enrichment is equally important for zebrafish welfare – they will almost exclusively choose an enriched environment over a barren one if given the opportunity, as seen in Video 1. Even when the enrichment provided is as simple as a picture of gravel or sand placed at the bottom of the tank, zebrafish will still prefer that over a barren tank.
Video 1: Zebrafish show a strong preference for enriched (left) versus barren (right) spaces.
In her talk, Lynne explained how the type of enrichment zebrafish prefer varies depending on their sex and place in the tank hierarchy. Group-housed male zebrafish show a strong preference for gravel, whereas group-housed females show a strong preference for sand. Sand is the most preferable enrichment for the dominant fish in a pair, but not the subordinate, which may be because the subordinate fish is behaviourally excluded from sand by the dominant. Instead, subordinate fish show a preference for floating artificial plants, which aren't as appealing to their dominant mates [1]. Providing your zebrafish with grass-like plants can also significantly increase the number of eggs they lay [2].
Lynne also spoke about how fish can perceive pain, similar to other commonly-used laboratory animals. If your fish behave abnormally after an invasive procedure, such as fin clipping, this may indicate that they are in pain – Lynne’s research has shown that providing analgesia can prevent these behavioural changes and therefore improve animal welfare (Videos 2a, 2b and 2c) [3]. Monitoring behaviour effectively is key for identifying when fish are in pain, so Lynne's lab has developed the Fish Behaviour Index (FBI) tool, which can monitor zebrafish activity continuously and non-invasively. The FBI automatically tracks fish movement and detects changes in welfare-relevant parameters like swimming speed, distance travelled, acceleration and time spent in specific areas. The system automatically generates alerts when it detects abnormal activity, enabling early intervention to improve welfare. Although the FBI can currently only monitor the behaviour of singly-housed fish, Lynne’s lab has recently developed a new tool, the chromatic fish analyser (CFA), which can examine the behaviour of zebrafish housed in groups.
Videos 2a, 2b and 2c: FBI video recordings of healthy fish (top), fish that have been fin-clipped (middle), and fish fin-clipped but given analgesia (bottom).
Lynne’s talk highlighted many things worth considering when you are working with zebrafish, including the appropriate use of enrichment and the effective detection of pain – demonstrating that welfare is just as important for fish as it is for other laboratory animals.
Training techniques for less stressed laboratory rodents
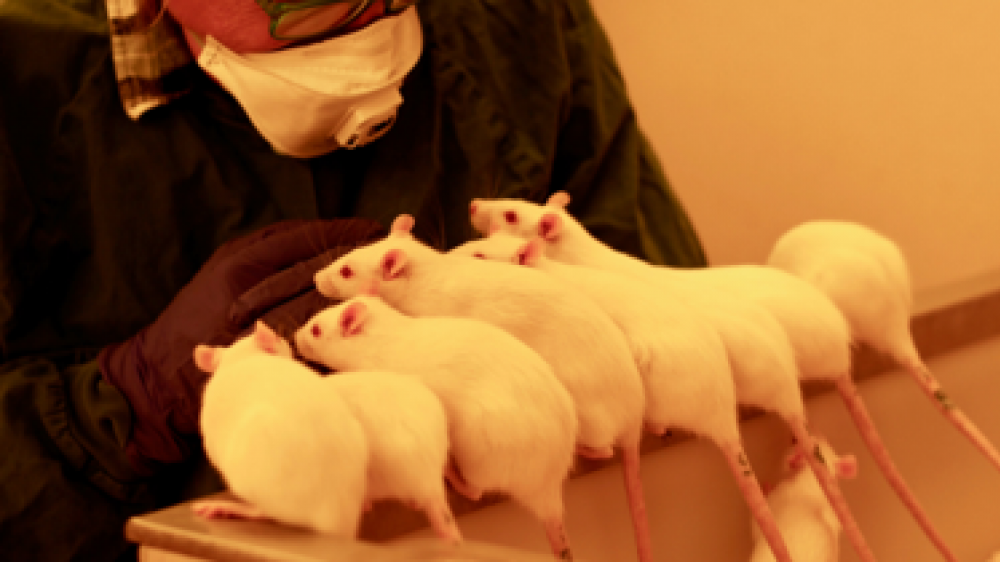
Environmental enrichment is not the only way to improve animal welfare in the lab. Positive interaction with technicians and researchers can also act as dynamic enrichment and significantly increase animal welfare. Ms Marie Eriksson is a project leader in RISE (Previous SWETOX), a network of research and technology organisations across Sweden. In her talk, she shared her expertise on how rodents become less fearful of humans through effective handling and training (Figure 1) and more cooperative when undergoing scientific procedures, which has a positive impact on both the animals and the technicians.
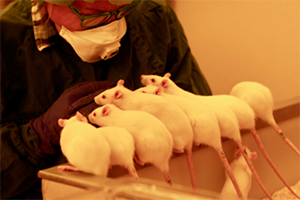
Figure 1: Rats appear fearless and seek interaction with the technician.
Video 3: A mouse that has been acclimatised to be handled by a technician and has been trained to undergo tail vein blood sampling, can be handled without the need for restraint and without any obvious stress or discomfort.
Videos 4a and 4b: Saphenous bleeding of an untrained (left) and trained (right) mouse. The untrained mouse is agitated, flipping a stiff tail, struggling and trying to get away. Its ears are flipped back, and eyes half shut. It is hard for the handler to find the blood vessel as the blood flow is "turned off". In contrast, the trained mouse is calm with a relaxed tail and open eyes. The mouse's colour is normal, and it is easy for the technician to find the blood vessel and fill up the capillary tube.
Rats can also be trained to undergo unpleasant procedures with the same benefits for both animals and technicians, including tail vein blood collection. This reduces or removes the need for restraint, with the animals appearing calm and cooperative during the whole process (Video 5). Marie explained how a cosy mat or blanket can be used as a positive reinforcement during the training process and the actual procedure, helping animals feel more secure and relaxed (as shown in Videos 3, 4 and 5). In the case of oral gavage, training can also decrease the cost associated with the procedure by reducing drug waste and damage to the gavage needle.
Video 5: A rat that has been trained to undergo tail vein blood collection. Following training and acclimatisation, the rat appears calm and cooperative and dosen’t require restraint during the procedure.
Marie also made the point that while training initially takes time and patience, the welfare benefits make it worthwhile. She recommended that technicians start with gentle handling to get animals used to their presence and gain their trust (Video 6) before moving onto more complex tasks. Training doesn’t have to focus on only painful and distressing procedures, but can also cover simple daily activities such as teaching animals to move from their transport box to their home cage independently (Video 7).
Video 6: Gentle and patient handling is required during the first days of training to gain the animals’ trust.
Video 7: Trained rats are used to the presence of the technician and even follow her as she moves around, seeking interaction. They have been trained to return to their transport box and then into their home cage by themselves, and therefore they don’t need to be individually picked up by the technician.
Once rats are used to the technician's presence, they will start actively seeking interaction by moving towards them and performing playful behaviours such as jumping, nipping and chasing the technician’s hands (Video 8). Similar behaviours have also been observed in trained mice, even though they tend to be more timid and skittish by nature than rats (Figure 2, Video 9).

Figure 2: Mice confidently and voluntarily interacting with the technician following habituation and training.
Video 8: Rats following the hand of the technician and performing playful behaviours in her presence.
Video 9: Mice appear calm and fearless, seeking interaction with the technician after habituation and training.
Marie’s experience shows that laboratory rodents can be trained to become less fearful of procedures and more relaxed when interacting with technicians. With patience and consistency, training can have a significant positive impact on the confidence and stress levels of both the animals themselves and the technicians that work with them.
Tickling rats for better welfare
Building on Marie’s talk, PhD student Megan LaFollette took rat handling and dynamic enrichment one step further by talking about how tickling rats can improve their welfare [5]. Megan explained that rats enjoy rough-and-tumble play from a very young age, and how engaging in it can have a positive effect on their emotions and stress levels. She also described how rats perform different vocalisations depending on their affective (emotional) state: one low-frequency vocalisation of around 22 kHz communicates negative emotions and is produced when rats experience pain or distress; another high-frequency call of around 50 kHz communicates positive emotions and occurs during play or when offered food treats [6]. When researchers mimic rats’ rough-and-tumble play by flipping, pinning and tickling them, rats produce these 50 kHz ultrasonic vocalisations (Video 10), which shows they enjoy being tickled by humans. After tickling sessions, rats are also more willing to approach technicians and show signs of decreased stress.
Video 10: Rats produce 50kHz vocalisations when tickled by a technician (turn up the volume to hear the rats’ high frequency vocalisations).
Tickling is particularly beneficial for rats that need to be singly housed due to the experimental setup and can, to an extent, satisfy their need for play and social interaction. It can also make rats calmer and easier to work with during painful or stressful procedures, similarly to Marie’s training approach. If you are new to rat tickling, you should practise on stuffed rats or try tickling young or pre-tickled rats. Ideally you should start tickling rats one to two days after they arrive at the animal facility, while they are still young and play is a natural behaviour for them. If you are concerned about extra workload or lack of time, Megan’s research has shown that even 15 seconds of tickling for three days is enough to significantly benefit rat welfare [7].
You might find that some rats do not seem to enjoy tickling initially. According to Megan, it is normal for different rats to have different responses to tickling, but it is best not to give up straight away. Instead, try tickling the rats again to see if they enjoy it more as they get used it. It is important to learn how to "read" your rats so you know when to stop if they don’t enjoy being tickled – look out for behaviours like actively avoiding your hand, freezing or tail rattling. Conversely, if your rats do 'joy jumps', try to lick or nibble your fingers or chase your hand, you can be confident that they enjoy being tickled. You can also use a simple bat detector or ultrasonic microphone to listen for positive (50kHz) or negative (22kHz) vocalisations during tickling.
Megan shared three key steps for good tickling:
- Initiate play by performing a light, brisk dorsal contact on the rat's nape (back of the neck) with one hand for two to three seconds (Video 11).
- Lift the rat properly using the correct grip (Figure 3). Make sure you hold the rat loosely but firmly and get used to manipulating its body weight without causing pain or discomfort.
- Confidently flip and pin the rat on its back and tickle its belly for three to four seconds, before letting it flip onto its belly again to start a new round of play (Video 12).
Video 11: Initiate play by lightly and briskly touching the rat’s nape.
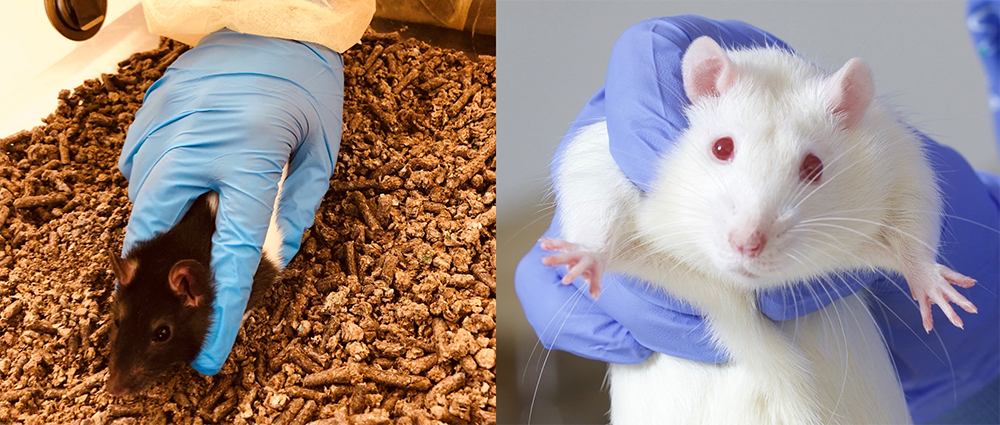
Figure 3: The incorrect (left) and the correct (right) way of gripping the rat before flipping and tickling.
Video 12: Flip, pin and tickle the rat's belly.
If you want to learn more about rat tickling, Megan has created an online rat tickling course containing videos, tutorials and useful tips on how to tickle your rats. If you pass all the quizzes, you can get a personalised Rat Tickling certificate at the end!
Poster session prize winners
As well as talks from experts in refinement and laboratory animal welfare, sixteen technicians from thirteen institutions presented their 3Rs-focused work during a flash poster session at the Symposium. The quality of the research presented this year was impressive: we heard about a range of new approaches technicians have implemented in their facilities to improve the welfare and reduce the use of a variety of species, from mice and rats to frogs and axolotls.
A £100 voucher prize was awarded to the best poster, based on the quality of the work, its 3Rs impact and the poster's overall presentation. The winner of this year’s prize was Nicola Cook from Fera Science for her work on introducing refinements to improve the care and welfare of their CAX mouse colony. Group-housed male CAX mice can be very aggressive towards each other, and female mice show increased aggression towards their male partners when paired up for mating. In an attempt to minimise aggressive behaviour, and inspired by the NC3Rs Mouse Aggression Study, Nicola introduced a number of changes to the housing and enrichment provided to these mice. She tied empty yogurt bottles to the cage lids to provide the mice with shelter high in the cage, where they naturally prefer to hide, and also introduced different food products weekly to encourage sensory and foraging activities. She also decreased the frequency of cage cleaning, which is known to cause stress in mice, from weekly to fortnightly and trialled different cage types that provided the mice with more living space. Finally, to minimise aggression in breeding pairs, male mice were removed from the breeding cage once pregnancy was confirmed. Ultimately, Nicola observed a sharp decrease in aggression, with four times fewer aggressive incidents recorded in the four-week period following the introduction of the above refinements, leading to a significant improvement in animal welfare. The poster judges were impressed by Nicola's comprehensive, successful approach to tackling the aggression problem as well as the overall clarity of the poster.

Figure 4: Nicola Cook (Fera Science), winner of the poster competition.
Due to the high quality of the posters presented this year, two runners-up were also chosen: Tom Leivers from the University of Nottingham and Joe Warmsley and colleagues from University College London.
Tom’s poster provided an overview of the University of Nottingham's strategy to implement non-aversive mouse handling methods. He shared his top tips on successfully rolling out the tunnel handling method across the University, which included having a proper understanding of the correct technique, using the free training resources from the NC3Rs and establishing good lines of communication, both with researchers and other animal care staff, so all can work together and share their observations and experiences.
Joe’s poster focused on ways to improve zebrafish husbandry. He has looked at how different parameters – namely diet, frequency of breeding, and a pathogen-free environment – can affect fecundity and fertility. His observations so far have shown that when zebrafish breed in pathogen-free conditions, they display higher fertilisation rates. He has also observed that frequent (weekly) breeding results in the production of fewer embryos over time compared to less frequent (monthly) breeding. Joe’s work demonstrates that improved understanding of how different factors affect zebrafish husbandry can refine breeding methods and reduce breeding populations.
Figure 5: Tom Leivers, University of Nottingham (top), and Joe Warmsley, University College London (bottom), winners of the runner-up awards.
Thanks to everyone who attended the Symposium, and to the Institute of Animal Technology for co-organising.
References
-
[1] Schroeder P et al. (2014). What do zebrafish want? Impact of social grouping, dominance and gender on preference for enrichment. Laboratory Animals 48(4): 328-337. doi:10.1177/0023677214538239
[2] Wafer LN et al. (2016). Effects of environmental enrichment on the fertility and fecundity of zebrafish (Danio rerio). Journal of the American Association for Laboratory Animal Science 55(3): 291-294. PMCID:PMC4865689
[3] Deakin AD et al. (2019). Automated monitoring of behaviour in zebrafish after invasive procedures. Scientific Reports 9, 9042. doi:10.1038/s41598-019-45464-w
[4] Thomson JS et al. (2019). Assessment of behaviour in groups of zebrafish (Danio rerio) using an intelligent software monitoring tool, the chromatic fish analyser. Journal of Neuroscience Methods 328: 108433. doi:10.1016/j.jneumeth.2019.108433
[5] Cloutier S et al. (2018). Tickling, a Technique for Inducing Positive Affect When Handling Rats. Journal of Visualized Experiments (135), e57190. doi:10.3791/57190
[6] Burgdorf J et al. (2008). Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. Journal of Computational Psychology 122(4): 357-367. doi:10.1037/a0012889
[7] LaFollette MR et al. (2018). Practical rat tickling: Determining an efficient and effective dosage of heterospecific play. Applied Animal Behaviour Science 208(11): 82-91. doi:10.1016/j.applanim.2018.08.005
