Evolution of behavioural monitoring technologies: the role of multidisciplinary collaborations
The ability to automatically monitor the behaviour and activity of animals that are socially housed with minimal disruption offers significant welfare and scientific benefits. The growth of this field and development of new technologies have been accelerated through the work of multidisciplinary collaborations. Here we describe some of the key activities in the field of behavioural phenotyping and highlight the TEATIME initiative for researchers to support the progression of the field.
Contents
- Abstract
- Introduction: the evolution of home-cage monitoring and behavioural testing
- Applications of home-cage monitoring systems in laboratory animal science
- Advances in technology and data analysis
- Broadening the scope of home-cage monitoring for metabolic studies
- Communicating the 3Rs impacts of behavioural monitoring to the public
- The COST TEATIME initiative
- Future perspectives
- Further reading
Abstract
Behavioural phenotyping of laboratory animals has been revolutionised through technological advances in home-cage monitoring and analysis systems. Advances in data storage, image analysis and deep learning techniques, in combination with miniaturisation and sophistication of hardware components, allow behavioural tests to be performed inside home-cages without disturbing the animals’ environment, delivering better quality scientific data alongside improvements in animal welfare. Critical in driving this progress have been interdisciplinary collaborations such as CRACK IT and platforms for knowledge exchange between multiple communities such as the TEATIME COST Action consortium, that bring together diverse expertise to deliver user-driven technologies. Consortia like the TEATIME COST Action are working towards the standardisation of protocols and analyses to support the production of robust, reproducible data, and to build capacity for the adoption of behavioural phenotyping technologies more broadly within the bioscience community. This article summarises the progression of behavioural phenotyping technology, focusing on its use in rodent studies. We describe key collaborations and opportunities for further multidisciplinary cooperation that may provide routes of access for researchers, technicians and other users.
Introduction: the evolution of home-cage monitoring and behavioural testing
Measuring the activity and behaviour of laboratory animals provides key information in preclinical research, including the progression of disease symptoms and indicators of the efficacy and safety of novel therapeutics. Classically, these measurements are taken by either monitoring the behaviour of individually housed rodents in tasks such as wheel running or removing the animals from their home-cage to study behavioural indicators in specific tests that can expose a potential scientific effect. These approaches have been standard practice for many years but have significant limitations, such as:
- Single housing and/or the removal of animals from their home-cage can cause significant stress, impacting welfare.
- Stress can also affect behaviour, which may impact the quality and types of scientific data obtained.
- Studies often take place during researcher working hours and may not provide an accurate reflection of the normal behaviour of rodents, which are typically active at night.
Early development of automated systems for home-cage monitoring included specialist cages for tracking activity but these still required animals to be singly-housed, leading to social isolation and stress that may affect their behaviour. Although these systems could monitor the dark phase during the night, the scope of behavioural studies was limited by the type of technology available and a lack of methods for the analysis and interpretation of the large quantities of data generated.
There was a clear unmet need for the measurement of activity in group-housed animals using an automated, minimally invasive approach to enable improvements in welfare and behavioural phenotyping. Integrating behavioural assessment with automated home-cage monitoring technologies requires expertise from various disciplines, including electronics, informatics and software development, as well as from life science researchers and animal technicians. The NC3Rs invested £1M through the two CRACK IT Challenges, Rodent Big Brother and its successor Rodent Little Brother, to address this need and support the development of a technology to record the activity, behaviour and temperature of individual rodents while group-housed in their home-cages.
CRACK IT Challenges
CRACK IT is the NC3Rs challenge-led innovation funding competition. The CRACK IT programme provides a unique opportunity for developers to form consortia and collaborate with potential end-users to address a specific scientific need to deliver real-world 3Rs impacts. This format supports the development of tools by technical experts from multiple sectors that are fit-for-purpose, promoting uptake by the life science community. To learn more about CRACK IT Challenges, please visit the NC3Rs Innovation Platform.
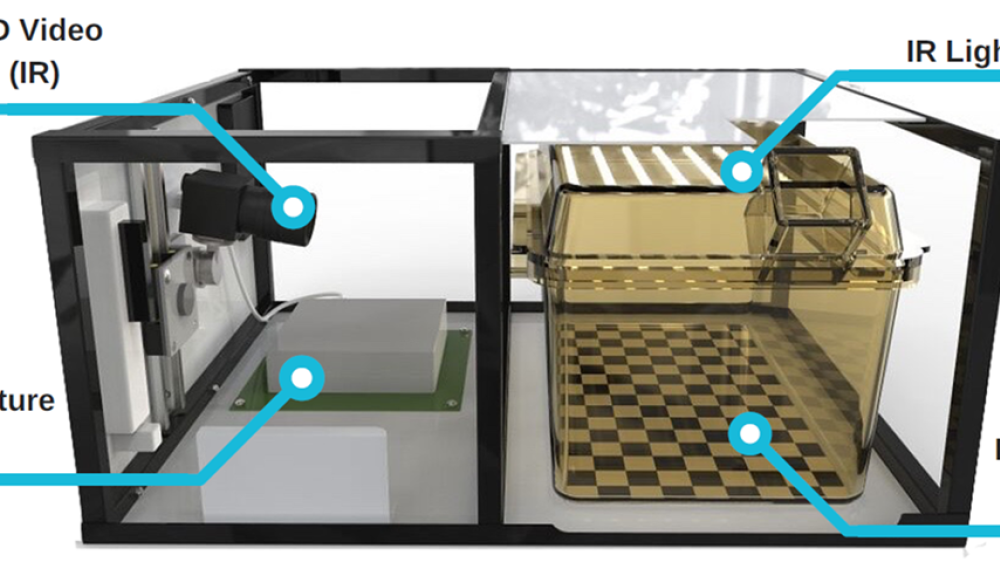
The Rodent Big Brother Challenge aimed to develop an automated, non-surgical system that could be used in rats and mice for the measurement of activity and temperature over a 24-hour period. The multidisciplinary team, led by Actual Analytics and supported by industry Sponsors Astra Zeneca, developed the Home Cage Analyser (HCA), a rodent home-cage behavioural monitoring system. The subsequent Rodent Little Brother Challenge, sponsored by MRC Harwell, further developed the HCA system for use with multiple mice in their home-cage setting. The HCA is compatible with individually vented caging (IVC) systems and automates the collection of spatial data over time using high-definition infrared video capture and radio-frequency identification of individual animals [1]. This enables the investigation of numerous parameters including locomotion, social separation and temperature data of individual rodents in a group-housed setting [2, 3]. Importantly, automated, non-invasive monitoring allows the incorporation of behavioural measurements into existing study types, from basic research through to drug discovery and development, thereby reducing the number of separate studies needed while increasing the potential for discovery through detailed analysis of continuously recorded behaviour. These capabilities have been recently demonstrated in a study of behavioural phenotypes in a mouse model of Huntington’s disease. Using the HCA, researchers observed that activity levels of the mouse model in light and dark phases reflect the early changes in circadian rhythms associated with Huntington’s disease in humans [4].
The first set of published data from the HCA system was derived from three commonly used background mouse strains and showcased, in an industry first, the unique ability to accurately discriminate between individuals within a single IVC cage of group-housed animals over multiple light-dark cycles. Over a 24-hour light-dark cycle, statistically significant differences in activity and duration of active periods were observed between strains [1]. These observations highlighted the variability of ‘typical’ mouse behaviour, as behaviour changes with strain, age, time of day and sex. These data were made publicly available to other research groups around the world for use in further research or as the basis to improve experimental design, such as to calculate the power of their experiments.
A number of next-generation automated home-cage monitoring systems incorporating advanced imaging technology, data analytics methods and machine learning algorithms, such as the HCA, are now commercially available and can provide a wealth of data supported by a greater understanding of its meaning and relevance to the phenotypes under investigation [5].
Applications of home-cage monitoring systems in laboratory animal science
Mice are naturally active in the dark phase and lighting conditions and time of day are known to influence their behaviour. Aligning training periods with the animals’ actual active phase has been shown to improve the success rate of training by providing mice with opportunities for voluntary learning in the dark phase. By attaching an operant chamber to the home-cage with gated access to allow a single animal into the chamber at a time, training time for operant tasks can be significantly reduced [6]. In addition to being a valuable scientific tool with improved task sensitivity, this approach also provides a form of enrichment, refining the task whilst reducing the number of mice required to obtain robust and reproducible data.
Home-cage monitoring studies also greatly enrich time-sensitive drug studies by capturing the temporal aspects of the treatment by increasing the reproducibility of temporal data collection across laboratories, particularly where there is an acute element to the effect [7, 8]. The use of home-cage monitoring for passive observation allows the same group of animals to be continuously monitored throughout a study, reducing the use of multiple cohorts in studies that have a high burden of testing. This approach may capture subtle indications of changes in welfare that may be missed through ‘snapshot’ testing, substantially refining the methodology, which is likely to improve the power of each experiment in preclinical research [4].
The ability to track multiple animals within a single home-cage has enabled the investigation of biologically relevant, non-evoked, voluntary social interactions. In addition to the analysis of phenotypes, investigation of group behaviours such as mating and pre-aggressive behaviours can inform better husbandry practices. One such example is the use of ultrasonic vocalisations (USVs) recorded over 24 hours within a home-cage, developed as a part of an NC3Rs-funded Fellowship (AVERT: Acoustic Vocalisation Early Response Technologies). Investigations into mouse USVs, together with behavioural traits, may provide interesting information on the social behaviour and welfare of mice that could further refine research practices and lead to the earlier prediction of adverse effects including disease onset [9]. The use of complementary technologies alongside automated home-cage monitoring will allow multiple aspects of behaviour to be monitored in a more quantitative, longitudinal and comprehensive way.
It is important to emphasise that such systems, although extremely sophisticated, are not a substitute for welfare assessment by trained animal technicians and the responsibility of care for laboratory animals remains with the staff and research scientists. However, the knowledge gained from these systems can be used as a training resource and to support animal technicians in making informed decisions. Early identification of adverse events will inevitably lead to improvements in earlier intervention and improved humane endpoints.
Advances in technology and data analysis
Successful development of the HCA technology highlighted the impact of home-cage monitoring systems and indicated an appetite in the life science community for commercially available systems that can combine video and spatiotemporal data and identify individual animals under group-housed conditions [3]. The applications of the HCA system highlighted in this article are summarised in Table 1.
The field has since developed considerably as relevant technologies have advanced and become more accessible to non-technical experts. Well-established deep learning techniques for image analysis have become more widely available, leading to a radical improvement in the design of behavioural tests and allowing image analysis to be applied to voluntary behaviours, which will have broad applications in the field. For example, in conditions such as stroke and amyotrophic lateral sclerosis, automated analysis of motor coordination and gait function has enriched the parameters that can be measured from a mouse walking voluntarily and has made traditional methods, such as subjective scoring of gait anomalies and the use of ink stains to chart the gait of an animal on a paper walkway, redundant [10].
The ability to process large amounts of information using artificial intelligence has reduced the time between data capture in passive monitoring studies and the analysis and interpretation of biologically meaningful data. New bioinformatics tools, such as those that will enable controlled behavioural ontologies, big-data analysis, and automated quantification and interpretation of observed behaviours, facilitate cross-comparison of results from different systems and laboratories. The ultimate goal of automated behavioural assessment is the characterisation of the full behavioural repertoire of a rodent under group-housed conditions, which is a complex task. Home-cage monitoring is an emerging field and to achieve this ambition the community must work together to develop complementary protocols and common formats for data analysis and interpretation, in order to maximise the potential of the diverse range of technology available.
Broadening the scope of home-cage monitoring for metabolic studies
In 2019, the MOSHERS CRACK IT Challenge was launched, with the aim of developing an approach or device to provide scalable, real-time 24/7 measurement of individual food intake in group-housed mice in a standard IVC home-cage. Conventional methods used to measure body weight and food intake in metabolic studies, involving handling and single housing, may exacerbate anxiety-like traits in mouse models and artificially alter food intake. MOuse Smart HoppERS (MOSHERS) were developed by Research Devices Ltd [12] to continuously measure food intake, feeding habits and cyclical patterns in mice using image analysis techniques. Data captured using these advanced technologies can inform future experiments involving food restriction, weight monitoring and/or mouse models of metabolic disorders. Metabolic phenotyping with MOSHERS also allows researchers to investigate the effect of interventions such as altered diets and/or drug treatment within the home-cage, minimising the effects of rodent anxiety and repeated experimenter intervention.
Communicating the 3Rs impacts of behavioural monitoring to the public
The Rodent Big Brother Challenge was an industry first, demonstrating the value of embracing emerging technologies and community-led approaches to problem solving. In 2018, the Rodent Little Brother project continued to embody these philosophies by enlisting the help of the public to analyse hundreds of minutes of video footage recorded to develop the HCA system. In a first-of-its-kind citizen science project, volunteers labelled mouse behaviours from video clips to support researchers study such behaviours in social settings across various time periods. The Rodent Little Brother: Secret Lives of Mice project, hosted by the Zooniverse platform, won an Openness Award in 2020 [11] for starting an open and honest conversation with the public about the importance of the 3Rs in animal science.
The COST TEATIME initiative
The success of home-cage monitoring systems and progression in the field of automated behavioural assessment relies on collaboration between sectors, data sharing and engaging in open conversation. To support this and encourage the adoption of these technologies in preclinical research, the European Cooperation in Science and Technology (COST) Action: Improving biomedical research by automated behaviour monitoring in the animal home-cage (TEATIME) was launched in 2021 [13].
The COST programme funds collaborative research networks, termed COST Actions, with the aim of fostering research advancements and innovation. The COST TEATIME Action was founded by 58 researchers in 23 countries, bringing together European organisations developing and using automated home-cage monitoring technologies. One of its primary goals is to expand the initiative to a network of 500 behavioural research scientists, manufacturers, bioinformaticians and experts in machine learning to form a collaborative, multidisciplinary consortium.
The project aims to provide a forum to share protocols and results that are as interpretable as those currently obtained from behavioural observations of animals outside their home-cage. Despite being considered the gold standard, such results can be affected by animals being in unfamiliar or variable environments. One of the first undertakings by the consortium has been to survey the scientific community to assess its requirements, identify outstanding scientific questions and determine any barriers to uptake [14].
The TEATIME project was designed to use the latest cutting-edge resources in machine learning to automatically detect biologically relevant voluntary behaviours within the home-cage. Before the project launched, hundreds of hours of video data required manual annotation in triplicate to allow the design of a machine learning algorithm able to automatically detect behaviours in a video. Now, such approaches are delegated to large deep learning networks that can detect multiple behaviours with minimal human intervention, allowing a whole new field of scientific investigation. Most importantly, such techniques can be applied in a retrospective, system-agnostic way without the need for breeding or using new animals, maximising the power of existing data and aligning closely with the ethos of reduction and refinement.
The training and information sharing encouraged and facilitated by this COST Action, for example through webinars, technology catalogues and training schools, has already led to increased standardisation for the use of home-cage monitoring systems among consortium members. These resources are targeted to improve reproducibility and robust experimentation that will support the wider adoption of these technologies and implementation of the 3Rs.
COST Action: Improving biomedical research by automated behaviour monitoring in the animal home-cage (TEATIME)
The TEATIME consortium aims to improve how animal behaviour is performed and investigate ways to develop the use of 24/7 monitoring using methods such as videos and sensor tracking for behavioural monitoring. Together, the consortium will:
- Address issues such as the diversity of equipment available, complementarity of protocols, and common formats for analysis and presentation of results to enable results to be more cross-comparable.
- Assess current needs regarding development of new bioinformatics tools, such as:
- Ontologies (a form of controlled vocabulary) to describe behaviours.
- Bioinformatics for the analysis of large volumes of data.
- Machine learning to automatically describe or quantify behaviours to reduce time spent watching lengthy videos.
Key activities include:
- Comparison of current state-of-the-art and future requirements for home-cage monitoring systems.
- Exploration of experimental design and parameters measured in member laboratories to curate baseline data.
- Development of a common understanding and communication of evaluation results to stakeholders and the wider research community, including best practice.
- Encouraging knowledge exchange by creating forums for multidisciplinary groups.
- Support the drive towards the production of reproducible data between laboratories.
The ultimate goal is to be able to describe animal behaviours with a minimum impact on the animals, by monitoring them their home-cages, enabling them to exhibit routine behaviours in a familiar environment.
Future perspectives
The emergence of home-cage monitoring systems over the past decade, ranging from those developed in-house to fully integrated, commercially available systems, has led to an increased understanding of mouse behaviour and demonstrates the benefits of these capabilities in preclinical research. Such systems provide a vital resource for informing better husbandry and welfare practices which in turn will improve scientific outcomes. As the field flourishes and increasing numbers of systems emerge to address specific scientific and husbandry needs, it is important to remain focused on the reproducibility of the data across laboratories. Initiatives such as TEATIME provide an open platform for data sharing and multidisciplinary collaboration, providing important support for the growth of the field and for critically assessing the potential of behavioural phenotyping technologies to improve preclinical research.
Further reading
Table 1: Overview of publications demonstrating the capabilities of the HCA
|
Publications describing HCA use |
Summary |
|---|---|
|
Bains RS et al. (2016) [1]. Analysis of Individual Mouse Activity in Group Housed Animals of Different Inbred Strains Using a Novel Automated Home Cage Analysis System. |
Introduction of the HCA system and reporting of individual locomotor behaviour in socially housed mice. |
|
Refern WS et al. (2017) [2]. Automated recording of home cage activity and temperature of individual rats housed in social groups: The Rodent Big Brother project. |
Development and validation of the HCA system, including initial observations of changes to activity and temperature caused by routine perturbations such as a cage change. |
|
Hobson L et al. (2020) [9]. Phenotyping in Mice Using Continuous Home Cage Monitoring and Ultrasonic Vocalization Recordings. |
Detailing a protocol for the continuous recording of home-cage activity using the HCA system and a protocol to continuously record ultrasonic vocalisation in group-housed mice. |
|
Mitchell EJ et al. (2020) [8]. Temporal dissociation of phencyclidine: Induced locomotor and social alterations in rats using an automated homecage monitoring system – implications for the 3Rs and preclinical drug discovery. |
Detection of behavioural deficits associated with a model of schizophrenia in rats, validating the HCA system to detect changes in social behaviour and locomotion, and highlighting the advantage of home-cage monitoring for long-term analysis (e.g. over the course of days). |
|
Wotton JM et al. (2022) [7]. Identifying genetic determinants of inflammatory pain in mice using a large-scale gene-targeted screen. |
Use of the HCA to support the investigation of mouse models of nociception, by recording and analysing changes in the behaviour of single-gene knockout mouse strains. |
|
Bains RS et al. (2023) [3]. Longitudinal home-cage automated assessment of climbing behavior shows sexual dimorphism and aging-related decrease in C57BL/6J healthy mice and allows early detection of motor impairment in the N171-82Q mouse model of Huntington’s disease. |
Demonstration of the HCA to analyse the phenotypic profile of a mouse model of Huntington’s disease over multiple light–dark cycles, including changes to climbing behaviour, in group-housed mice. |
References
- Bains SB et al. (2016). Analysis of Individual Mouse Activity in Group Housed Animals of Different Inbred Strains Using a Novel Automated Home Cage Analysis System. Frontiers in Behavioral Neuroscience. 10. doi: 10.3389/fnbeh.2016.00106
- Refern WS et al. (2017). Automated recording of home cage activity and temperature of individual rats housed in social groups: The Rodent Big Brother project. PLoS ONE. 12(9) doi: 10.1371/journal.pone.0181068
- Bains RS et al. (2018). Assessing mouse behaviour throughout the light/dark cycle using automated in-cage analysis tools. Journal of Neuroscience Methods. 15(300). doi: 10.1016/j.jneumeth.2017.04.014.
- Bains RS et al. (2023). Longitudinal home-cage automated assessment of climbing behavior shows sexual dimorphism and aging-related decrease in C57BL/6J healthy mice and allows early detection of motor impairment in the N171-82Q mouse model of Huntington’s disease. 17. doi: 10.3389/fnbeh.2023.1148172
- Baran SW et al. (2022). Emerging Role of Translational Digital Biomarkers Within Home Cage Monitoring Technologies in Preclinical Drug Discovery and Development. Frontiers in Behavioral Neuroscience. 15. doi: 10.3389/fnbeh.2021.758274
- Caglayan A et al. (2021). The Stop Signal Task for Measuring Behavioral Inhibition in Mice With Increased Sensitivity and High-Throughput Operation. Frontiers in Behavioral Neuroscience. 15. doi: 10.3389/fnbeh.2021.777767
- Wotton JM et al. (2022). Identifying genetic determinants of inflammatory pain in mice using a large-scale gene-targeted screen. Pain. 163(6) p. 1139-1157 doi: 10.1097/j.pain.0000000000002481
- Mitchell EJ et al. (2020). Temporal dissociation of phencyclidine: Induced locomotor and social alterations in rats using an automated homecage monitoring system – implications for the 3Rs and preclinical drug discovery. Journal of Psychopharmacology. 34(7) p. 709-715 doi: 10.1177/0269881120920455
- Hobson L et al. (2020). Phenotyping in Mice Using Continuous Home Cage Monitoring and Ultrasonic Vocalization Recordings. Current protocols in mouse biology. 10(3), p. e80. doi: 10.1002/cpmo.80
- Preisig DF et al. (2016). High-speed video gait analysis reveals early and characteristic locomotor phenotypes in mouse models of neurodegenerative movement disorders. Behavioural Brain Research. 311. doi: 10.1016/j.bbr.2016.04.044
- NC3Rs (2020). 3Rs citizen science project wins at the 7th Annual Openness Awards
- NC3Rs (2019). NC3Rs Innovation Platform Challenge 31: Moshers
- COST (2021). CA20135 - Improving biomedical research by automated behaviour monitoring in the animal home-cage (TEATIME)
- Hölter SM et al. (2022) Improving biomedical research by automated behaviour monitoring in the animal home cage – action needed for networking. Laboratory Animals. 0. doi: 10.1177/00236772221117451
Learn more about technologies and tools that allow the automatic monitoring of animal behaviour and activity.