Refinement of rodent and non-rodent housing during telemetry recordings
At a glance

Overview
Telemetry is a method which allows the remote measurement of physiological parameters from animals in their home environment without disturbance. Telemetered rodents (guinea-pigs, rats and mice) are often used within research models or early drug screening. Non-rodents (dogs, mini-pigs or non-human primates) are required for cardiovascular safety studies of potential new medicines before human clinical trials, designed to better understand the potential of drugs to affect parameters including blood pressure, heart rate and ECG. This is commonly performed during either a standalone safety pharmacology study (with the animals implanted with a telemetry device) or as part of the repeat dose toxicology study (using recordings obtained from animals wearing jackets containing the recording equipment, or from implanted telemetry devices).
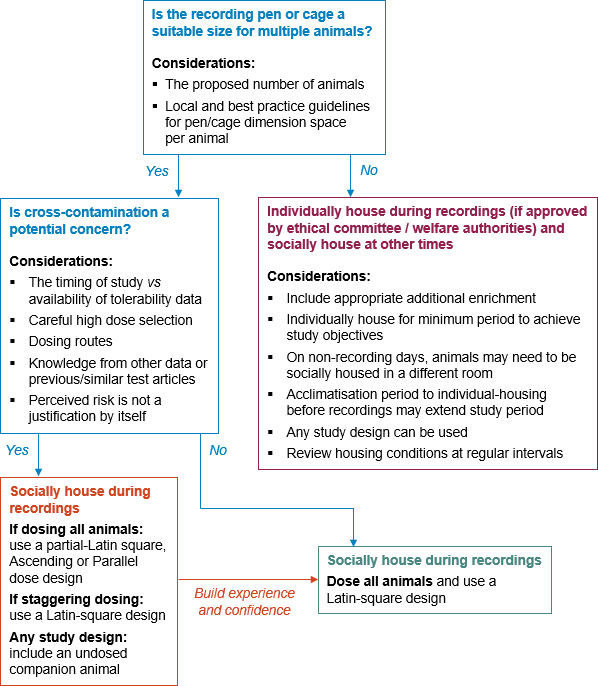
During these studies, animals were often individually-housed due to cross-talk between signals. However, in the last decade, developments in telemetry technologies has allowed recordings from animals housed in compatible pairs or groups (social housing). The NC3Rs has worked with the pharmaceutical industry to promote adoption of this refinement, conducting surveys in 2015 and 2017 to better understand company approaches and the opportunities and barriers for adopting best practice in social housing. A workshop was held at the Safety Pharmacology Society 15th annual meeting in Prague (2015) and a break-out session within a NC3Rs cardiovascular showcase event in 2018, where experience was shared between different companies to encourage wider implementation.
Evidence of enhanced data quality and similar responses to a range of positive control substances have been published for rats [2] and non-rodents [1, 3]. This latest publication [1] provides recommendations for best practice study designs taking other common barriers to social housing into account (e.g., pen or cage sizes only suitable for a single animal, or concerns over cross-contamination between animals in the same pen receiving different dose levels).
Publications
Papers
- Prior H and Holbrook M (2021). Strategies to encourage the adoption of social housing during cardiovascular telemetry recordings in non-rodents. Journal of Pharmacological and Toxicological Methods 108: 106959. doi: 10.1016/j.vascn.2021.106959
- Skinner M et al. (2019). Social-housing and use of double-decker cages in rat telemetry studies. Journal of Pharmacological and Toxicological Methods 96: 87-94. doi: 10.1016/j.vascn.2019.02.005
- Prior H et al. (2016). Social housing of non-rodents during cardiovascular recordings in safety pharmacology and toxicology studies. Journal of Pharmacological and Toxicological Methods 81: 75-87. doi: 10.1016/j.vascn.2016.03.004
Posters
- Poster: Social housing during telemetry studies in rodents and non-rodents. Poster NC3Rs Pharma showcase 2019.
- Poster: Why are non-rodents not socially housed during cardiovascular telemetry recordings on safety pharmacology studies. SPS 2017.
- Poster: Global cross-company data-sharing on the housing of non-rodents during the recording of cardiovascular telemetry data on safety pharmacology studies. SPS 2015.
- Poster: Global cross-company data-sharing on the housing of non-rodents during the recording of cardiovascular telemetry data on toxicology studies. EuroTox 2015.
Bibliography of external resources
Papers
- Markert M et al. (2018). A new telemetry-based system for assessing cardiovascular function in group-housed large animals. Taking the 3Rs to a new level with the evaluation of remote measurements via cloud data transmission. Journal of Pharmacological and Toxicological Methods 93: 90-7. doi: 10.1016/j.vascn.2018.03.006
- Andersen N et al. (2017). Evaluation of the PhysioTel Digital M11 cardiovascular telemetry implant in socially housed cynomolgus monkeys up to 16 weeks after surgery. Journal of Pharmacological and Toxicological Methods 87: 82-92. doi: 10.1016/j.vascn.2017.04.007
- Cordes J et al. (2016). Validation and utility of the PhysioTel Digital M11 telemetry implant for cardiovascular data evaluation in cynomolgus monkeys and Beagle dogs. Journal of Pharmacological and Toxicological Methods 79: 72-9. doi: 10.1016/j.vascn.2016.01.00
- Sadekova N et al. (2016). The effects of housing conditions on baseline cardiovascular parameters and the sensitivity to detect changes in contractility in telemetry-implanted dogs. Journal of Pharmacological and Toxicological Methods 81: 60-74. doi: 10.1016/j.vascn.2016.05.001
- Xing G et al. (2015). Effects of group housing on ECG assessment in conscious cynomolgus monkeys. Journal of Pharmacological and Toxicological Methods 75: 44-51. doi: 10.1016/j.vascn.2015.05.00
- Kaiser R et al. (2015). Telemetric assessment of social and single housing: Evaluation of electrocardiographic intervals in jacketed cynomolgus monkeys. Journal of Pharmacological and Toxicological Methods 75: 38-43. doi: 10.1016/j.vascn.2015.05.001
- Hawkins P (2014). Refining housing, husbandry and care for animals used in studies involving biotelemetry. Animals 4: 361-373. doi: 10.3390/ani4020361
- Markert M et al. (2012). Evaluation of a method to correct the contractility index LVdP/dt(max) for changes in heart rate. Journal of Pharmacological and Toxicological Methods. 66:98-105. doi: 10.1016/j.vascn.2012.04.005
- Stubhan M et al. (2008). Evaluation of cardiovascular and ECG parameters in the normal, freely moving Gottingen minipig. Journal of Pharmacological and Toxicological Methods 57: 202-211. doi: 10.1016/j.vascn.2008.02.001
- Markert M et al. (2007). The value added by measuring myocardial contractility in vivo in safety pharmacological profiling of drug candidates. Journal of Pharmacological and Toxicological Methods 56: 203-211. doi: 10.1016/j.vascn.2007.03.004
- Klumpp A et al. (2006). Optimising the experimental environment for dog telemetry studies. Journal of Pharmacological and Toxicological Methods 54: 141-149. doi: 10.1016/j.vascn.2006.03.010
Posters
- Poster: Impact of housing conditions on cardiovascular parameters in telemetry-implanted cynomolgus monkeys. SPS 2019.
- Poster: Evaluation of PhysioTel digital M11 cardiovascular telemetry implant in socially-housed cynomolgus monkeys using Etilifrine and Moxifloxacin. SPS 2019.
- Poster: A novel freely moving animal-based blood pressure, ECG and dual body temperature telemtry system for group housed mice in social context. NC3Rs cardiovascular showcase 2018.
- Poster: Continuous real-time physiological data from freely moving group-housed animals. NC3Rs cardiovascular showcase 2018.
- Poster: Leadless heart rate loggers minimize impact of surgery and allow social-housing. NC3Rs cardiovascular showcase 2018.
- Poster: Advances in physiologic telemetric monitoring compatible with social housing. NC3Rs cardiovascular showcase 2018.
- Poster: Improvement of animal welfare as well as robustness of cardiovascular data, how New Telemetry Technologies and study designing can support. NC3Rs cardiovascular showcase 2018.
- Poster: Gottingen minipigs pre-implanted with DSI PhysioTel digital telemetry implants. NC3Rs cardiovascular showcase 2018.
- Poster: Cardiovascular parameters in socially housed cynomolgus monkeys. JSPS 2017.
- Poster: Comparison of telemetric cardiovascular parameters in single and pair housing of cynomolgus monkeys. SPS 2016.
- Poster: A comparison of baseline HR, LV and SBP in group vs single housed dogs and the effect of housing on sensitivity to detect changes in contractility. SPS 2015.
- Poster: Comparative analysis of data sciences international PhysioTel D70 and PhysioTel digital telemtry platforms. SPS 2015.
- Poster: Evaluation of digital implantable telemetry in multiple social housing paradigms for cynomolgus monkeys. SPS 2015.
- Poster: JET-BP in socially housed non-human primates, comparison of Covance sites and study considerations. SOT 2015.
- Poster: Pair-housed dog telemetry, animal welfare refinement with early indications of similar study sensitivity. SPS 2014.
- Poster: Effect of moxifloxacin hydrochloride on cardiovascular parameters assessed via jacketed external telemetry (JET) in the male beagle dog co-housed in European caging. SOS 2014.
- Poster: Pimobendan, etilefrine, moxifloxiacine and esketamine as reference compounds to validate the DSI PhysioTel system in cynomolgus monkeys. SPS 2013.
- Poster: Assessment of drug-induced cardiovascular effects by telemetry in group-housed cynomolgus monkeys. SPS 2013.
- Poster: The use of the new PhysioTel digital implant (DSI) for cardiovascular assessment in the Gottingen minipig. SPS 2012.
- Poster: Non-invasive telemetry for monitoring ECG in singly and group housed dogs - the effect of moxifloxacin. SPS 2008.
