Tech3Rs: Issue 17 (May 2023)
In this edition we share how gentle handling and proper habituation leads to low stress procedures for mice and rats. We also highlight two Tech3Rs champions making refinements for zebrafish, webinar recordings on evaluating environmental enrichment and microsampling, and the new-look macaque website.
In this issue
Handling and habituation of mice and rats for low stress procedures

A must-watch webinar for anyone working with rodents in research.
Creating trust and cooperation between research animals and handlers improves animal welfare and study reliability by decreasing animal stress during scientific procedures. In a webinar hosted by the NC3Rs, Therése Ahlström, a laboratory engineer and animal technician, spoke about the pioneering approach taken at Research Institutes of Sweden (RISE) to refine procedures for mice and rats. Therése highlighted the importance of refining all aspects of research animals’ lives, touching upon enriched housing and socialisation before focusing on the importance of consistent gentle handling and proper habituation.
Gentle handling includes taking a refined approach to picking up mice (e.g. using a tunnel), but Therése and her colleagues go beyond this to make all interactions with their mice and rats as positive as possible, even for large-scale studies. All rodents are habituated to handling in five sessions over a two-week acclimatisation period before the start of a study. Each session last one to two minutes at a time and involves calmly and confidently handling the rodents with a soft and gentle approach, including picking them up and stroking them.
To prepare the mice and rats for what will happen during the study repeated contact is made with body parts that will be handled as part of procedures. For example, if the tail vein will be used to collect blood the tail is routinely stroked and gently manipulated before the study commences. The mice and rats are also familiarised with equipment, such as electric shavers used to remove hair. To build a positive association with being handled the rodents are rewarded with treats and kept comfortable using fleecy autoclavable bedding, which is also provided for comfort during the later experimental procedures.
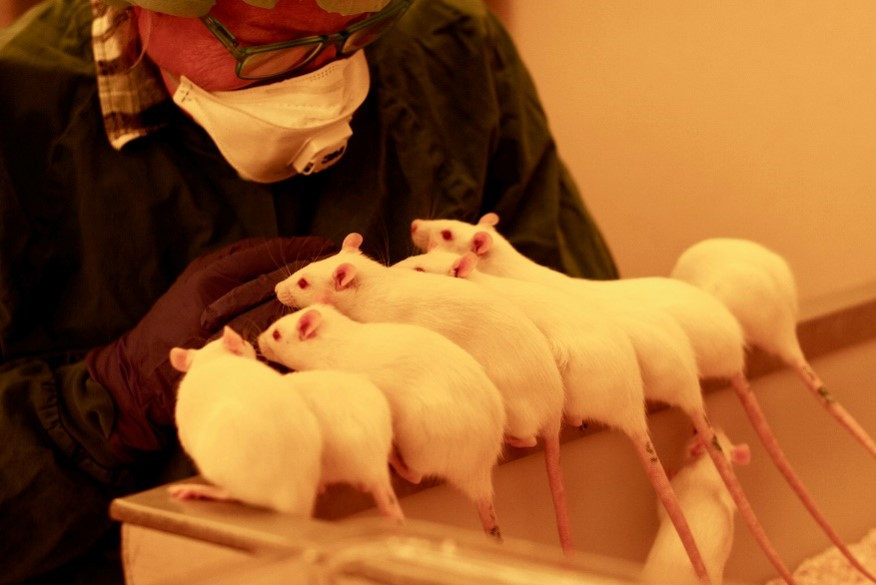
Ultimately these short sessions aim to reduce the distress associated with human handling and aversive procedures, and to build trust between the animals and handlers. The powerful videos shared by Therése show that the initial time invested in preparing the rodents for studies is highly effective and worthwhile. The mice and rats willingly interact with staff and appear calm, cooperative and largely unphased during sampling and administration, which take place with minimal to no restraint.

Therése and colleagues report that their approach facilitates more accurate observations following dosing, with a reduced risk of confusing stress-related behaviors with substance-related behaviors. An additional benefit is the impact on staff wellbeing, working with calm, confident and cooperative animals has the potential to greatly reduce handler stress. As is often the case when implementing refinements, Therése says that when you change your way of working you won’t want to go back.
To see the impressive results that arise from this approach and learn more about how these refinements are applied to a range of different sampling and dosing procedures, watch the webinar recording: Handling and training of mice and rats for low stress procedures.
Revisit the 2019 NC3Rs/IAT Animal Technicians’ Symposium highlights.
The approach taken by RISE for low stress procedures with mice and rats
- Gentle handling starts as soon as the animals arrive at the facility, beginning when they are unpacked.
- Mice and rats typically have five habituation sessions before the start of the study, lasting only one to two minutes each.
- Sessions always end with something positive, such as a treat or a gentle cuddle for rats.
- The study protocol informs which body parts receive additional handling and what equipment the animals are familiarised with.
- During session one the rodents sit on comfortable fleecy bedding and are gently stroked.
- During sessions two to five the animals get used to anything they will encounter in the experiment, such as new environments and equipment.
- During the final session the rodents should be calm and curious.

Tech3Rs champion: Haley Gatzogiannis
Haley Gatzogiannis is a Vivarium Operations Supervisor at Novartis Institutes for BioMedical Research, USA. She spoke to us about switching from fin clipping to skin swabbing for DNA sampling of zebrafish.
What 3Rs idea have you implemented?
I work with transgenic zebrafish and part of my day-to-day role involves collecting DNA samples from the fish for genotyping (identifying their genetic make-up). Previously, our standard approach was to take fin samples from the zebrafish (fin clipping). We have now implemented a refined and less invasive method of collecting DNA from zebrafish. Instead of fin tissue, we sample the mucus layer on the flank of the fish, which contains shed skin cells. The mucus swab samples can then be used in a very similar way to how fin samples are for DNA sequencing. We now have an official protocol in place for collecting DNA samples from zebrafish by skin swab, processing the samples, and amplifying the DNA for in-house sequencing to give us accurate genotyping results.
How did you develop this approach?
We have regular 3Rs group meetings to ensure that we are staying up to date and carrying out procedures in the most refined way. My colleagues made me aware of a refined technique for genotyping zebrafish. They had come across information on the swabbing technique in the literature and the NC3Rs website. I thought it was a great idea, and something we could easily attempt. The initial appeal to me was that it was less invasive than fin clipping without the need for anesthesia or tissue removal. My colleagues and I set out to investigate whether we could obtain adequate DNA concentrations for quality sequencing results.
To avoid the use of live zebrafish while we familiarised ourselves with the swabbing technique, we obtained cadavers through ongoing research projects. This avoided the use of any additional fish while allowing us to humanely investigate whether we could get quality swab samples from the mucus layer. To make direct comparisons, we took two samples from each zebrafish cadaver – a biopsy sample of fin tissue and a swab of the mucus layer. We found that the fidelity of the swab samples was good with a 100% match between fin and skin cell DNA results, validating this approach for successfully genotyping our zebrafish. One way we optimised our sample collection technique was to change from a typical cotton swab to a microbrush, also called a microapplicator. The microbrush was less absorbent than the cotton swab and fit well in a 96 well plate, which was convenient as that is what we use for DNA samples.

It was a learning curve for us to find the best and most efficient way to restrain the anaesthetised fish, but we have a team that provides support for new approaches, and I worked closely with them on this to develop a methodology that is efficient and minimally distressing to the fish. We use a wetted sponge, gloved hands, and a net.
What impact has this project had on you?
Our top priority was improving the DNA sampling technique to benefit the animals, so it feels great to have worked as a team to achieve this. Approximately 22% of all the animals needed for our R&D are zebrafish, and while not all these fish will be genotyped, this refinement will still positively impact a significant number of animals.
Building on our success at the US site, I shared our new approach with our Swiss colleagues, and they are exploring adoption of the swabbing technique too. Implementing this refinement and sharing it with our international colleagues led our team to be awarded the 2022 Novartis Global 3Rs Award for Refinement.
My advice to anyone looking to make an impactful change for the animals in our care would be collaboration – this project included input and feedback from many different groups at our site. Our zebrafish knowledge, combined with our Animal Welfare and Veterinary experts, Scientists, and Novel Technical Procedures team worked together to achieve this refinement.
Tech3Rs champion: Diane Fleary-Jones
Diane Fleary-Jones is an Animal Technologist at the University of Birmingham. She spoke to us about evaluating environmental enrichment for zebrafish.
What 3Rs idea have you developed?
I have undertaken an enrichment trial to investigate which types of structural enrichment our zebrafish prefer and whether these have an impact on egg laying. The data I collected as part of my small-scale study is being used to inform change within our unit, where we have around 200 tanks of zebrafish. With the support of my colleagues, an enrichment item that I designed will soon be introduced in our zebrafish tanks as standard.
This item will be an addition to the evidence-backed environmental enrichment that we currently provide to our zebrafish: a gravel sticker underneath each tank, a commercially available plastic plant and live food. Zebrafish have been shown to prefer gravel stickers and plants to barren tanks, and live food stimulates natural predatory behaviour [1]. I was motivated to carry out my own evaluation to take an informed approach to choosing new in-tank structural enrichment.
How did you develop this project?
I used published information and my own experiences working with and caring for fishes, including zebrafish, to design different structures that I thought the zebrafish would engage with. I constructed structural enrichment items inspired by natural aquatic habitats. This included a surgical drape designed to mimic floating weeds; a plastic divider with two holes for the fish to swim through; and a system of cable ties anchored to a stick at the top of the tank to mimic fronds extending down into the water.

I worked with our NC3Rs Regional Programme Manager, Dr Nicola Foster, to design and carry out an evaluation to answer the questions: ‘which enrichment item do the fish interact with the most?’ and ‘do the enrichment items impact egg laying?’. Certain in-tank structures can cause zebrafish to lay eggs spontaneously. This is something we need to avoid when controlling the timing of egg laying for research purposes so it was important that we collected data on this.
An additional challenge with zebrafish is deciding what to measure to determine if enrichment items are improving their welfare. We made the assumption that the zebrafish will choose to interact with items that they prefer, and that this interaction indicates a positive impact onwelfare. We also monitored behaviours that could indicate a negative effect of the enrichment on welfare, such as aggressive interactions.
What was the outcome of the project?
The data that I collected from the enrichment trial was presented at our 3Rs Focus Group meeting. We did not see any increase in indicators of negative welfare for any of the enrichment items evaluated. The fish were observed interacting with the cable tie ‘fronds’ more frequently than the other items, for example, by swimming through them. There was also minimal disruption to egg laying with this enrichment item. These factors led us to conclude that, from the options presented, the cable tie enrichment was the best for zebrafish welfare and our own research purposes.
When discussing the project as a group, concerns were raised about plastic from the cable ties leaching into tank water. We decided to use a material that is already approved for use in aquatics, rather than cable ties, to make a similar structure. We settled on commercially available flexible tubing and are currently waiting for this to arrive before we can roll-out the new enrichment to all zebrafish tanks.
Being creative, coming up with new ideas and observing the results was really interesting. Following this project I have an increased confidence in how to evaluate environmental enrichment and am proactively looking for more opportunities to do so.
Key tips for evaluating zebrafish enrichment
- Communicate with your colleagues and get permission before you start planning an evaluation.
- Determine how many groups you need and set up the appropriate number of tanks – be sure to include a control group with no enrichment or with just the enrichment you already use.
- Check that any materials you are using do not leach or interfere with the water flow.
- Include the enrichment that you already use in the evaluation – animals can respond differently to enrichment if is combined with other items.
- Give the animals time to acclimate to the new enrichment.
- Monitor the effects on egg laying, as researchers will want to see this data.
- Photograph/video everything. Give the fish time to adjust to any disturbance before filming.
- Rotate the items around each group to make sure it isn’t just a preference in an individual group (or one fish).
References
From Tech3Rs to the Andrew Blake Tribute award
Congratulations to former Tech3Rs champion Alicia Kinally on winning the IAT Andrew Blake Tribute award for her refinements to rabbit handling. In Issue 15 of Tech3Rs Alicia, an animal technician at the University of Leicester, shared her approach to improving the daily capture of rabbits. Alicia’s project focused on encouraging rabbits to voluntarily hop into a pet carrier, which has now replaced hands-on capture when removing the animals from their cages.

Read Alicia’s interview in Tech3Rs Issue 15, page 4 nc3rs.org.uk/Tech3Rs.
For video guidance on handling and lifting rabbits, including footage of Alicia transporting a rabbit using the method described here, visit our rabbit housing and husbandry page.
Updates from the NC3Rs
Webinar recording: Microsampling in toxicology – Maximising the scientific, business and 3Rs advantages
In collaboration with the British Toxicology Society (BTS), the NC3Rs hosted a webinar to raise awareness of the microsampling technique and encourage wider adoption in toxicology. Microsampling is an approach where only a very small volume of blood is taken from an animal, typically ≤50 μl. This procedure is quicker and less stressful and can reduce the number of animals used in a study.
Six talks covering different topics are available to watch online, including Dr Hollie Blunt’s (Sequani) presentation on the animal welfare benefits of microsampling for mice, rats, rabbits, dogs and minipigs.
View the talks to learn more about microsampling: Microsampling in toxicology – Maximising the scientific, business and 3Rs advantages.
The macaque website
The macaque website is a free resource for everyone who works with, or is interested in, laboratory macaques. It contains information, videos and audio recordings providing practical guidance on the natural history and behaviour of macaques, their care and management in captivity, and ways to assess their welfare. Topics, include:
- Interpreting macaque facial expressions, postures and vocalisations.
- How social housing in large, enriched enclosures can save time, space and money.
- Designing and implementing enrichment programmes.
- Best practice husbandry, including how to approach habituation and training for different procedures.
- Behavioural, physiological and health indicators to inform comprehensive welfare assessments.
Visit the macaque website to learn more.
Evaluations of environmental enrichment: workshop and webinar update
We would like to say a big thank you to everyone who attended the NC3Rs workshop on evaluating environmental enrichment at IAT Congress 2023. Of the attendees who provided feedback 94% said they found the session extremely useful or very useful and 100% would recommend the workshop to other animal technicians.

To help us reach a wider audience and allow you to learn how to approach evaluations of enrichment we also ran a webinar that you can now watch online. Dr Khia Dobbinson (NC3Rs) shared some of the resources available to support animal technicians in evaluating enrichment, and Zoe Windsor (Senior Research Support Technician and NACWO, University College London) gave practical guidance from a technician’s perspective.
Watch the recording and read the session Q&A: Webinar: Evaluations of environmental enrichment.
Get an email notification as soon as the next issue of Tech3Rs is available to download. We recommend that this is then circulated to colleagues or printed out for display in animal houses.

Feature in a future issue!
- Send us an idea for a feature.
- Nominate a 3Rs champion.
- Tell us about a new 3Rs approach.
- Flag a 3Rs-focused paper.
Email us at tech3rs@nc3rs.org.uk.
