Increasing human tissue use
It is increasingly recognised that models using human tissue or cells can contribute to a better understanding of human health and disease and drive the development of safe and efficacious medicinal products. These models can also help to reduce the reliance on animals in research and underpin more translationally relevant testing strategies.
On this page
What is human tissue?
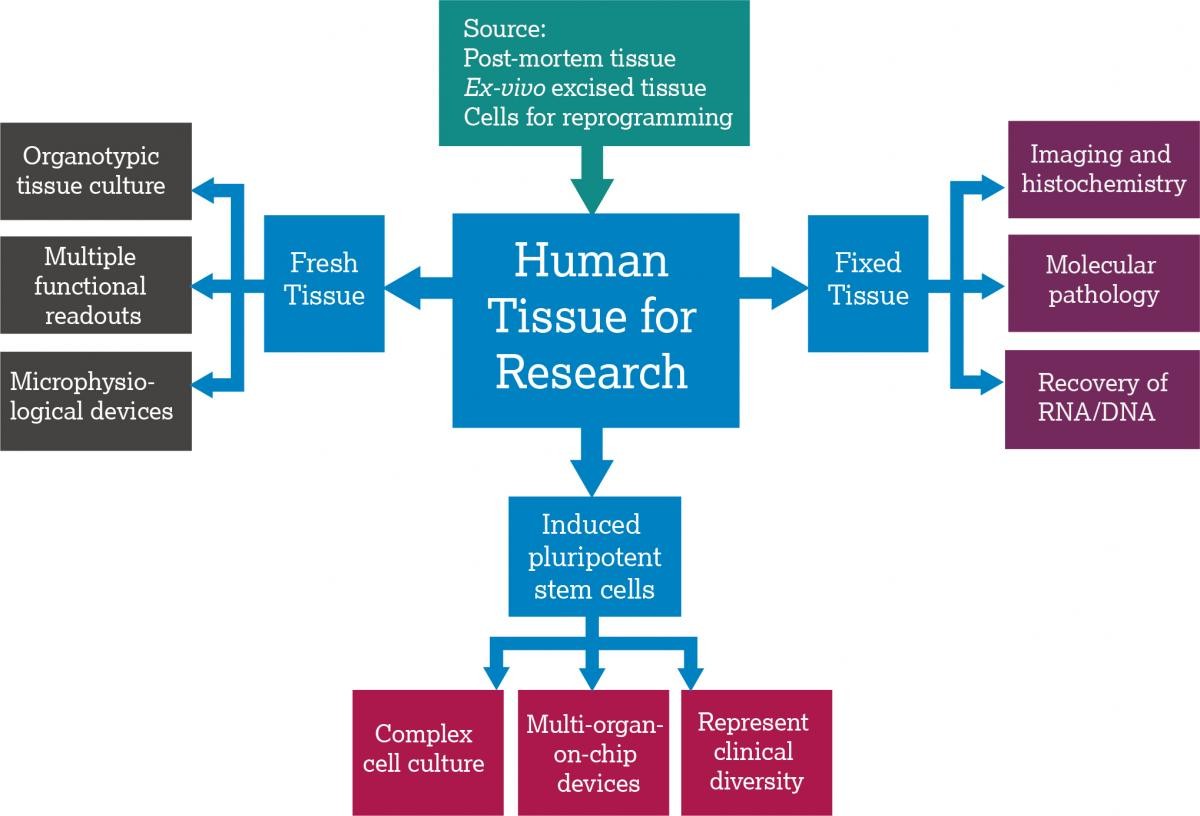
Ex vivo tissue: Human tissue from surgery or post-mortem sources, and human blood or fluids, can be used in research. Both private and publicly funded biobanking initiatives in the UK and EU are being developed to enable human tissue and metadata collection, storage and dissemination (e.g. UKCRC Tissue Directory and Coordination Centre in the UK and BBMRI-ERIC in the EU which are both publicly funded and can be used by researchers for free). Commercial biobanks are also supplying human tissue to pharmaceutical companies for use in these studies, and contract research organisations are offering out-sourced human tissue modeling.
Human stem cells: Recent advances in induced pluripotent stem cell (iPSC) technology and gene editing have expanded the definition of human tissue. The Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative is one example of a co-ordinated multinational approach to implementing iPSC-based models in safety assessment. iPSCs are often purchased from commercial suppliers, although non-commercial projects such as the European Bank for Induced Pluripotent Stem Cells (EBiSC) and STEMBANCC are now providing alternative sources.
What is the NC3Rs role?
Working closely with researchers from academia and industry, international regulatory agencies and other key stakeholders, we have embarked on a programme of work to explore, understand and address the barriers to increased human tissue use in research.
We have carried out surveys to map the human tissue modelling landscape, engaging the UK asthma research community and the global drug safety assessment community in order to better understand the extent of human tissue use and the potential barriers to wider adoption in these areas. The surveys identified similar key barriers, which include:
- Practicalities associated with acquisition and storage of human tissue.
- Access to a regular and sustainable supply of high-quality tissue with associated metadata.
- A confusing and complex regulatory landscape.
Improving access to human tissue
The most widely cited barrier preventing uptake of human tissue-based approaches is lack of access to a reliable source of tissue. However this is not considered a problem by all researchers and many consider that substantial amounts of human tissue are available in existing repositories and biobanks and from commercial sources; suggesting that a key issue is a lack of knowledge regarding what repositories exist.
There are a number of ongoing activities both in the UK and Europe to coordinate biobanking activity and provide researchers with a means to more easily identify and access collections of tissue relevant to them. The most coordinated of these is the UK Clinical Research Collaboration (UKCRC) Tissue Directory and Coordination Centre, which is the UK partner in the Biobanking and BioMolecular resources Research Infrastructure – European Research Infrastructure Consortium (BBMRI-ERIC). Another source of human tissue for research is the NHS Blood and Transplant service (NHS BT) which provides organs for transplant, but also supports scientists in accessing for research, organs which are not suitable for this purpose. Marketplaces for outsourced research services, such as Scientist.com, also offer opportunities for accessing tissue.
Who is Tissue and Eye Services?
NHSBT Tissue and Eye Services (TES) is a human tissue bank based in Liverpool. TES is the largest tissue bank in the UK and one of the largest in Europe, issuing 12,000 human tissue allografts each year to surgeons for transplantation purposes. TES is set up to retrieve tissue for both clinical and R&D use.
Supporting greater access to human tissue for research
TES can provide human tissue for ethically approved research either:
- From tissue taken for clinical use but then found to be unsuitable for transplantation – TES staff ask for research consent during the consent process or;
- From research donors who are unsuitable for clinical donation.
In both cases, consent can be obtained for specific tissue if required. Tissues are retrieved within 48 hours of death (24 hours for ocular tissue).
What tissues are available?
- Skin – cellular and decellularised
- Bone – whole, shaped, ground, demineralised
- Tendons
- Hearts and heart valves
- Arteries
- Eyes, cornea and ocular globes
- Amniotic membrane
- Many other tissues on request
Tissue can be supplied fresh, frozen, freeze dried or cryopreserved.
Helping to reduce the use of animals in research
Respiratory diseases (e.g. asthma and Chronic Obstructive Pulmonary Disease) are often uniquely human conditions, making it difficult to interpret data generated in animal models and apply the knowledge gained in a logical way to the human disease process.
TES has recently joined in a collaboration with Imperial College London (funded by the NC3Rs) to investigate the potential of using lung, trachea and vagus nerve tissue from diseased donors in research into respiratory disease.
TES will provide human tissue with full donor family consent at various times after death for evaluation in a series of well-established assays. The objective is to determine whether human tissue from deceased donors can be used successfully and to produce guidelines on the most appropriate conditions under which the tissue can be retrieved and used. The data obtained can be extended to other organ/tissue systems, thereby helping to further reduce the need to use animal-based assays in human disease research.
How can researchers get access to TES human tissue?
Researchers can apply to TES directly by contacting Paul Rooney (Research and Development Manager) at paul.rooney@nhsbt.nhs.uk
A sample list of tissue available can be found on the NHS Blood and Transplant website.
What is Scientist.com?
Scientist.com (formerly Assay Depot) is the world’s largest marketplace for outsourced research services. Launched in 2008, the marketplace is a procure-to-pay platform that saves researchers’ time, reduces costs, provides access to the latest innovations and ensures regulatory compliance. Our vision is to create a world in which scientists are limited only by their imagination.
Scientist.com operates private marketplaces for ten large pharmaceutical partners, a private research portal for the US National Institutes of Health and a Biotech marketplace for biotech and academic researchers. Researchers use the marketplaces to access 3,500 research areas offered by over 15,000 suppliers in 100 countries.
The platform’s unique capabilities allows qualified institutions to capture institutional knowledge, integrate internal finance and sourcing workflows, purchase high risk services (such as human samples) and track time and cost savings - while maintaining full compliance with your organization’s procurement policies.
Supporting greater access to human tissue for research
Although human biological samples (HBS) are increasingly being used in pharmaceutical research, there has been no standard compliance process governing their acquisition by researchers. The new Scientist.com HBS compliance process helps researchers and suppliers adhere to internal and external (often legislative) policies for HBS acquisition. -It also ensures that appropriate documentation has been obtained and communicated throughout the supply chain, and by focusing on donor consent it significantly reduces the risks associated with using HBS.
Created in partnership with eight leading pharmaceutical companies, leading HBS suppliers and Biobank organizations such as the UK Clinical Research Collaboration Tissue Directory and Coordination Centre (UKCRC TCC), the new compliance and governance framework increases visibility, traceability and control for both commercial and academic HBS sources. It standardizes the ways pharmaceutical companies and individual researchers evaluate potential Biobanks and by doing so simplifies the process and reduces costs for all parties. It is only available through the Scientist.com marketplace.
Interacting with Scientist.com
Scientist.com offers the opportunity for researchers to identify and access multiple sources of consented HBS from global suppliers (both commercial and academic) with which to conduct human relevant research programmes at scale.
Researchers can learn more and register for a free account at www.scientist.com/researchers
Commercial suppliers & Biobanks can learn more and register for a free supplier account at www.scientist.com/suppliers
Further information:
- Email: support@scientist.com
- Information Website: www.scientist.com
- User Website: http://app.scientist.com
- Supplier (Biobank) Website: http://backoffice.scientist.com
The UK Tissue Directory
The UKCRC Tissue Directory is an online catalogue of over 200 human sample resources in the UK (tissue banks, biorepositories, biobanks, cohort studies, clinical trials etc.).
The Tissue Directory enables a quick, free and efficient route for researchers to locate appropriate samples and data to match their research needs. Researchers can search the Directory for existing sample collections or organisations that can collect bespoke collections. It is possible to search by a specific disease term or ‘fit and well’. An A-Z of human sample resources is also available.
About the UKCRC TDCC
The mission of the UKCRC Tissue Directory and Coordination Centre (UKCRC TDCC) is to maximise the use, value and impact of the UK's human sample resources in the UK, and beyond. The UKCRC TDCC do this by creating a world-leading, research-enabling, and networked biobanking infrastructure to facilitate the discovery and use of the UK's human samples and data.
Run by a dedicated team across the University of Nottingham and University College London, the creation of the UKCRC TDCC was mandated by the UK Clinical Research Collaboration via their Vision for Human Tissue Resources. The UKCRC TDCC works to help researchers discover samples and data, help sample resources improve their data systems for sharing, and harmonise policy relating to the discovery and use of samples and data.
The work of the UKCRC TDCC is guided by the belief that the biomedical research ecosystem should be based on open standards, open-science, and pre-competitive collaboration.
The UKCRC TDCC also acts as the UK node for BBMRI-ERIC: working to promote quality and interoperability across Europe.
Further information:
- Email: contact@biobankinguk.org
- Telephone: +44 (0)20 3549 5849
- Twitter: @BiobankingUK
- LinkedIn: UKCRC Tissue Directory and Coordination Centre
- Website: biobankinguk.org
Who is Seralab?
Seralab, a division of BioreclamationIVT, is a leading provider of biological products to the life sciences and pharmaceutical industries throughout Europe. With ISO 9001:2015 accreditation, Seralab specializes in control and disease state matrices manufactured from human and animal biological specimens and has recently expanded their offering to include cell processing and phenotypic screening services.
Ethical sourcing of high quality biospecimens
We have a large, growing clinical network made up of over 100 physicians, research groups, hospitals, and reference labs that provide access to over 70,000 specimens.
What do you do/offer?
Seralab has the ability to collect fully customizable healthy, human products to meet specific customer protocols. This allows us to tailor each order according to your exact specifications while maintaining the highest level of product quality.
The portfolio includes a broad range of offerings, including:
- ADME-Tox Products: Hepatocytes, Subcellular Fractions, Processed Skin Tissue and Derivatives
- Cellular Products: PBMCs, Purified cell types (T cells, monocytes, CD34+, etc.), Splenocytes, Fibroblasts, Renal Cells
- Normal/Healthy and Disease State Fluids: Blood/Serum/Plasma, Bone Marrow, CSF, Amniotic fluid, Swabs
- Disease State Tissues: Normal, Adjacent and Tumor Tissue, FFPE, Tissue Microarrays, Skin Punch Biopsies
- Services: Cell-Based Assays, Clinical Lab Testing, Histology, Proteomic & Molecular Services
- Media: Human AB serum, FBS, Select Growth Factors and Stem Cell Research Reagents
Further information:
- Email: customerservice@seralab.co.uk
- Telephone: +44 (0)1444 250010
- Website: www.seralab.co.uk.
What is BDR?
Brains for dementia research (BDR) is more than a tissue bank, BDR is a network of five UK-based brain banks. In total over 3000 people have been recruited to BDR and include those living with mild cognitive impairment or a diagnosis of dementia and healthy volunteers all of whom take part in continued assessments prior to brain donation.
What material is available through the BDR?
BDR is a hugely valuable resource for dementia researchers allowing them to investigate the pathology and underlying mechanisms of dementia using brain tissue, cerebrospinal fluid, derived material as well as a range of data sets. BDR provides researchers with gold-standard human brain tissue along with comprehensive neuropathological and clinical data to support their studies. Further, UK-based studies using BDR are covered by our ‘generic approval’, meaning studies can proceed without requiring their own ethical approval.
Accessing tissue through the BDR
Applying for samples or data from BDR is straightforward and researchers from both academia and industry worldwide can request access. Please contact the coordinating centre or search the full list of BDR tissue available on the UK Brain Banks Network. Researchers can then apply using the form for the appropriate brain bank(s). Applications will be assessed through a peer review process. This flow chart illustrates the process.
Further information:
- Email: BDR.Coordinatingcentre@ncl.ac.uk
- Twitter: @brains4dementia
- Website: www.brainsfordementiaresearch.org.uk
If you have human tissue repositories accessible to researchers and would like to share these with the wider research community please let us know by contacting enquiries@nc3rs.org.uk.
We have also funded projects to increase access to human tissue for specific applications:
- A strategic collaboration between academics and NHS BT to develop a process to provide fully ethically consented, human normal and diseased lung tissue to the UK scientific community. If successful, the approach could apply to other organs and tissues.
- A CRACK IT Challenge to put in place a system to supply high quality and viable dorsal root ganglion neurones to researchers to facilitate drug target identification and pharmacological testing of novel pain therapeutics.
Navigating the regulatory and ethical framework
Working with the Human Tissue Authority, we have developed a series of decision trees to help guide researchers through the regulatory framework for accessing and using human tissue to help overcome the often cited barrier that this is complex and confusing. The decision trees cover what is considered relevant material, licencing for storage of human tissue and whether consent is required for research.
Other resources on this topic can be found on the MRC Regulatory Support Centre webpages.
Relevant Material: Determining if the human tissue you intend to use is considered relevant material (any tissue or sample containing human cells - some exclusions apply) and what licence you will need.
Storing human tissue: Navigating the licensing requirements for storing human tissue for research.
Consent: Determining if consent is required to use human tissue for research.
Download the decision trees:
Human tissue for safety pharmacology
An NC3Rs/MHRA project to increase the use of human tissue models for safety assessment of new drugs.
You can view the full details on our project page: Human tissue for safety pharmacology.
Human tissue in asthma research
Asthma is a uniquely human disease, making it difficult to interpret data generated in animal models and apply the knowledge gained in a logical way to the human disease process. Addressing this, we have a broad programme of work to develop alternative approaches to replace the use of animals and provide a better understanding of human asthma. This includes supporting the use of tissue engineering approaches, non-mammalian models (e.g. Drosophila, zebrafish), mathematical modelling and human tissue.
Barriers to human tissue use
Working with Asthma UK, the UK Respiratory Research Collaborative and the UK Human Tissue Authority we have surveyed the UK asthma research community to better understand the extent of human tissue use and the potential barriers to wider uptake.
The full survey data report and accompanying article in Thorax can be downloaded below, but it highlighted the potential impact greater use of human tissue could have on understanding the disease, but that access to reliable supplies of human tissue was not sufficient to meet demand. We have used the output from the survey to inform our activities in this area and provide resources and case studies to support researchers in their efforts to use human tissue as part of their asthma research programmes.
NC3Rs awards to increase access to human tissue
In 2016 we made an infrastructure award to Professor Maria Belvisi at Imperial College London to support the development of a process to provide fully ethically consented, post-mortem human normal and diseased lung tissue to the UK scientific community thereby reducing the need for animal tissue. The award is unique in that it includes a strategic partnership with NHS Blood and Transplant Tissue and Eye Services, the organisation responsible for coordinating, collecting, banking and providing tissue for transplant within the NHS. This partnership could truly transform access to human tissue for research purposes and although the initial focus is on asthma, the principles developed during this award will extend to other disease areas.
Working with members of our Asthma Advisory Group we provide below a series of case studies describing each of their different approaches to accessing human tissue to meet their research needs. These give a broad overview of the many ways human tissue can be sourced for research purposes and may guide you in your own efforts to adopt human tissue-based approaches.
Case studies

Professor Maria Belvisi is the head of the Respiratory Pharmacology group at the National Heart and Lung Institute, Imperial College London.
Maria is an internationally recognised expert and her research is focused on the cellular and molecular mechanisms of asthma, COPD and chronic cough. In 2010, along with Dr Mark Birrell, she formed IR Pharma; a preclinical respiratory drug discovery organisation which is part of the Imperial Innovations portfolio of companies.
There are a variety of animal models for studying conditions such as asthma and COPD, but none fully recreate the human condition. In order to develop effective new therapies it is essential to investigate mechanisms in human tissue.
We require large pieces of lung with the vagus nerve intact, but found that these are not easily accessible, posing a significant barrier to using this tissue for our experiments. We have taken two approaches to try to overcome this. Firstly, we purchase whole lung from a not-for-profit organisation, the International Institute for the Advancement of Medicine (IIAM), based in the US. IIAM work alongside not-for-profit organ procurement organisations (OPOs) to distribute human organs and tissue to researchers around the world.
The non-profit status means that we find it more financially viable to bring in tissue from the US than purchase from alternative commercial sources. We are able to make exact specification requirements regarding the tissue including disease status, age, and the time off a ventilator.
“Using human tissue has decreased animal use in our lab by 23%.”
In addition to this, in the UK we have registered to receive organ tissue with approval obtained for research use that is removed for transplantation, but is then rejected for that purpose - any researcher can request to receive this tissue. Each year there are currently approximately 400 lung samples where the tissue is not suitable for transplant but which could be used for research. However, due to the lack of infrastructure and information around access and distribution of samples, this is not utilised. Through an NC3Rs Infrastructure for Impact award, we are currently looking at ways to implement an OPO-style approach in this country, to ensure more human tissue can be made available for research purposes.
The main advice I would give to someone who wanted to access human tissue for research is to be flexible. Access is by far the most important barrier to overcome, but once you are able to access tissue it may become available at short notice and unsociable hours. For my researchers, when tissue becomes available then utilising this is a priority . In addition we share any tissue we receive with other labs to maximise the scientific and 3Rs benefit of any tissue we are given.
Using human tissue has had a huge impact on our research. We have seen an increase in our publication rate in translational respiratory journals since adopting human tissue approaches. This is in contrast to those that suggest publishing data generated in human tissue is difficult.
“We have seen an increase in our publication rate since adopting human tissue approaches.”
Additionally, our work is now more translatable to a clinical setting, and my lab was involved in generating key data sets during the development of tiotropium bromide (Spiriva), a long-acting muscarinic antagonist that is used in the treatment of COPD.
3Rs impact statement:
- Replacing some animal work with human tissue experiments has enabled us to reduce our annual animal usage by 23%.
- Demonstrating mechanistic proof of concept in relevant human vagal tissue samples has enabled the progression of new drugs for cough into clinical trials, and potentially reduced the need for some studies in animals.
- We have stopped using some animal models because data from human tissue has indicated that certain mechanisms do not operate in rodents.

Professor Peter Bradding is a Clinical Professor in respiratory medicine at the University of Leicester, based at Glenfield Hospital. Peter's research on the pathobiology of asthma, focuses in particular on ion channels and mast cell-airway smooth muscle interactions.
We have been using human tissue in our research for almost 20 years, and the system in place at our institution works very well.
“On average we receive suitable donor tissue twice a week.”
The main pieces of advice I would give are:
- Ensure the surgeons are on board. This is easier when you are located within the same building, but measures such as exploring mutual areas of interest for collaborative research are likely to be helpful.
- Ensure a close working relationship with the pathologists, and where possible, train laboratory staff so that they are able to remove samples from resected tissue for research when the pathologists are not available to help.
We have trained staff that monitor the scheduled operations list to identify any procedures that may result in usable tissue being removed.
Patients are then sent a study information pack, and usually consented for research the day before surgery. Most patients give consent, and this is highlighted prominently in their file. The surgeons automatically check for this consent authorisation whenever they are performing an appropriate operation.
The surgeons then remove the tissue, store it appropriately, and immediately contact our trained staff to collect the tissue. This is taken to the pathologists who remove what they need and pass the rest straight back to us.
“The training of our tissue technician by a pathologist ensures that lab research using human tissue doesn’t depend on the availability of the pathologists.”
Two members of staff have been trained by the pathologists to take what is needed for research immediately and leave enough for the pathologists for times when there is no pathologist available.
There have been no problems since we started doing this, and it ensures that lab research using human tissue does not depend on the availability of the pathologists.
"Important physiological differences exist between the mouse and human, and many key features of respiratory diseases are not reproduced in animal models. Additionally, mouse data are sometimes misleading and can hamper significant advances in respiratory medicine. The development of anti-IL-5 therapy is a good example.”
3Rs impact statement:
- As a result of being able to access human tissue so readily, we do not need to use animals for this research.

Professor Clive Page is Professor of Pharmacology, and Director of the Sackler Institute of Pulmonary Pharmacology at King’s College London.
Clive's research focuses on the pharmacology of inflammation and respiratory diseases. In 2006 he co-founded Verona Pharma plc, to develop new pharmacotherapeutics for respiratory diseases.
As asthma is a uniquely human condition affecting the lung, it is ideal to test potential new therapies in human tissue early in development.
We are carrying out two types of experiment that require human tissue. Biopsies and culture explants from diseased and healthy volunteers enable us to look at asthmatic vs. healthy lung, while access to whole lobes of lung enables us to study airways, and their response to drugs, in an organ bath setting as well as being able to perform video microscopy on the tissue.
“Work with human tissue has helped us to develop a drug to treat asthma and COPD which is now undergoing clinical trials.”
We found that our collaborators in Rome had much better access to larger sections of lung than we did. This is because most of the larger sections of tissue we use come from thoracotomies, where they remove a significant amount of healthy tissue from around the edge of the diseased area. More thoracotomies are carried out by the hospitals we have links to, than in those local to my lab in the UK, where they prefer less invasive diagnostic techniques.
This prompted me to situate one of my post-doc researchers in the city. He is able to collect tissue himself straight from the operating theatre and we get lung tissue weekly using this set up. Utilising tissue from the UK, as well as tissue from Italy has meant us having to deal with ethics and regulatory agencies in both countries, however obtaining the correct ethical approval and licences has been a very straight-forward process and we currently have ethics approval at three separate Italian hospitals, as well as the necessary licenses gained through the UK Human Tissue Authority.
“We have had ten publications resulting from our human tissue work.”
Many researchers have suggested that publishing human tissue-based research can be difficult, and cite this as a barrier to adopting this approach.
Our experience has been quite the opposite. We have published ten articles on our human tissue work over the last ten years, in journals such as American Journal of Respiratory and Critical Care Medicine, and the British Journal of Pharmacology.
Most importantly we have developed a new drug which is currently undergoing human clinical trials, in part based on studies with human lung tissues that predicted the efficacy of the drug in patients. Such work gave us a degree of confidence that we had discovered a drug that would offer benefit to patients, above and beyond the work we had carried out in laboratory animals.
3Rs impact statement
- Wider use of human tissue reduces the need for tissues from experimental animals and is therefore to be strongly encouraged.
