Future Vision: technology development for preclinical imaging and what it could mean for the advancement of the 3Rs

Continuing our focus on future technologies, we asked Professor Paul Matthews, from Imperial College London, how he thinks the advancement of preclinical imaging is likely to impact on the 3Rs.
Paul Matthews is Head of the Division of Brain Sciences at Imperial College, London and a Fellow by Special Election of St. Edmund Hall, Oxford. He received his training in at Oxford, Stanford and McGill as a neurologist and his research focuses on the relationship between microglial activation, adaptive plasticity and neuroaxonal loss as a mechanism of disability progression in multiple sclerosis.
I have been thinking about future developments for preclinical imaging and how in vivo imaging could have a greater impact on preclinical research, particularly for therapeutics development. A major part of my work has been in enhancing the informativeness of clinical imaging – to make humans the preferred experimental animal for clinical therapeutics research. But this will not allow us to learn all that we need to know.
One aspect of the challenge is to increase what we can learn from imaging studies. Technology development has repeatedly opened new windows onto biology through imaging – the advent of MRI, PET, ultrasound imaging, optical coherence tomography and optogenetics have each enabled step changes in the kinds of observations that can be made. What will the next major advance be? Nanomagnetics, “smart” sensors, Raman imaging? There is promise in many areas, but it is always impossible to be specific in predictions. The greatest impacts have come in the past from those developments we have not been able to see coming many years before.
What we can see are the continued improvements in current technologies and their application. Central to this has been more effective data integration through advanced data management and image analysis solutions. It already is becoming increasingly less common for multimodal examinations to be evaluated in anything other than a multimodal context.
One of the specific areas that I am especially excited about is the growth of optical imaging methods. Although there is no question but that optogenetics tools are transforming biology, let me highlight especially the promise of near infrared (NIR) optical imaging instruments and probes, which can be applied more generally and offer a potential for direct translation to human application. They already are becoming well-tested products. NIR enables deeper penetration for effective whole body imaging in smaller animals or deeper imaging in humans. The method can be applied with only modest training. An exciting extension is the use of awake, behaving and unconstrained animals in imaging studies. Reducing the need for restraint or anesthesia would simplify experiments, lower costs and contribute to improved animal welfare. I predict that we are going to see more use of these for dynamic measures of pathology and treatment responses. This could have a big impact on the severity of studies, as well as the numbers of animals that are used in experimental therapeutics.
A major driver for developing greater capabilities will be increasing incentives for innovation. Perhaps this will come with the greater emphasis on pharma outsourcing of early development projects in academia? Increased outsourcing offers an opportunity for academic groups to be rewarded for time spent in reducing new concepts to robust practice. It offers opportunities for new kinds of academic “spin out” clinical research organisations to be set up, as well, using well characterised, more widely used preclinical models (e.g., those for oncology or neuroscience) in optimised protocols for evaluations of major classes of therapeutics. Enhancing robustness of tools and protocols is an important step in the improvement of animal use.
A big challenge is to see the next step: how to help such businesses move from provision of “niche” services to the mainstream. This is important if they are to make the major investments needed for significant changes for more efficient process development and technology optimisation. While we are still some way from this in any general way, a major, addressable challenge is the development of standardised approaches for commonly addressed problems. Two areas have struck me as being particularly interesting in this regard: development of imaging based toxicology models, e.g., using transgenic reporter animals, or approaches for biodistribution studies of biologicals. To have the greatest impact for industry, regulators need to be brought on board. With their concerns – along with academia’s demands for scientific value and industries’ needs for robustness – concepts can be crystallised around standardisation of best methods to address major discovery and development problems so that regulators can develop confidence in the measures.
This vision sets some specific scientific goals. New types of generally informative measures are able to be applied across modalities, e.g., for general processes such as apoptosis, would be of value. Lowering the costs of tools through making tools more accessible to non-specialists (particularly MRI and PET) and refining instrument designs and increasing production to lower costs all would enhance uptake. We also need to improve our IT environments for managing, sharing and searching imaging data acquired across multiple modalities and species.
I am very optimistic about the future here. Over the last three decades that I have been watching the area, we have seen a continued evolution of capability in the field. I am confident that imaging science will continue to surprise us with what it can do.
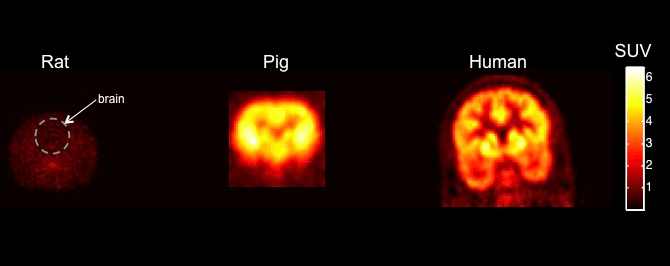
Figure 1: Shows PET biodistribution for a novel drug candidate in brains of rodent, pig and human. It highlights how where a molecule goes in the body can be evaluated with just a single animal, but also, in showing that a rodent does not show signal in brain, but a human does, how the human is the ideal experimental animal for many studies.
![Shows a {11C] acetate PET study before and after treatment in a mouse xenograft tumour, again highlighting how quantitative tumour data can be obtained with single animals able to be followed serially.](/sites/default/files/styles/max_1300x1300/public/2021-10/Fig%202.png?itok=rgpnAIeF)
Figure 2: Shows a {11C] acetate PET study before and after treatment in a mouse xenograft tumour, again highlighting how quantitative tumour data can be obtained with single animals able to be followed serially.
![Shows how [11C]PBR28 PET imaging- sensitive to inflammatory cells- can be used as a powerful tool for imaging the distribution of inflammation in the lung of a rat after inhalation of a lipopolysacchardie (LPS) challenge non-invasvively and on more than a single occasion, allowing effects of a drug to be assessed with just a single animal imaged at multiple times](/sites/default/files/styles/max_1300x1300/public/2021-10/Fig%203.png?itok=0u-24ywv)
Figure 3: Shows how [11C]PBR28 PET imaging- sensitive to inflammatory cells- can be used as a powerful tool for imaging the distribution of inflammation in the lung of a rat after inhalation of a lipopolysacchardie (LPS) challenge non-invasvively and on more than a single occasion, allowing effects of a drug to be assessed with just a single animal imaged at multiple times.
Images courtesy of the Clinical Imaging Centre, Hammersmith Hospital
