Re-evaluating the need for mAb chronic toxicity studies
At a glance
Contents
Overview
Under a European Partnership for Alternative Approaches to Animal Testing (EPAA) supported and funded project, a consortium including 14 pharmaceutical companies, the Medicines Evaluation Board (MEB) and the NC3Rs conducted a study to evaluate whether a 6-month toxicity study is still necessary to assess the long-term safety of mAbs.
The data collected for the project also enabled a review of study designs and current practices on animal use during mAb development and identification of opportunities to minimise the use of NHPs.

Webinar
On 17 April 2023, we hosted the webinar: Re-evaluating the need for mAb chronic toxicity studies, to provide an overview of the two publications from this project, discussing key results and recommendations.
The webinar is relevant for regulatory and industry scientists working with mAbs and other drug modalities interested in exploring new approaches for chronic toxicity studies and opportunities to apply the 3Rs.
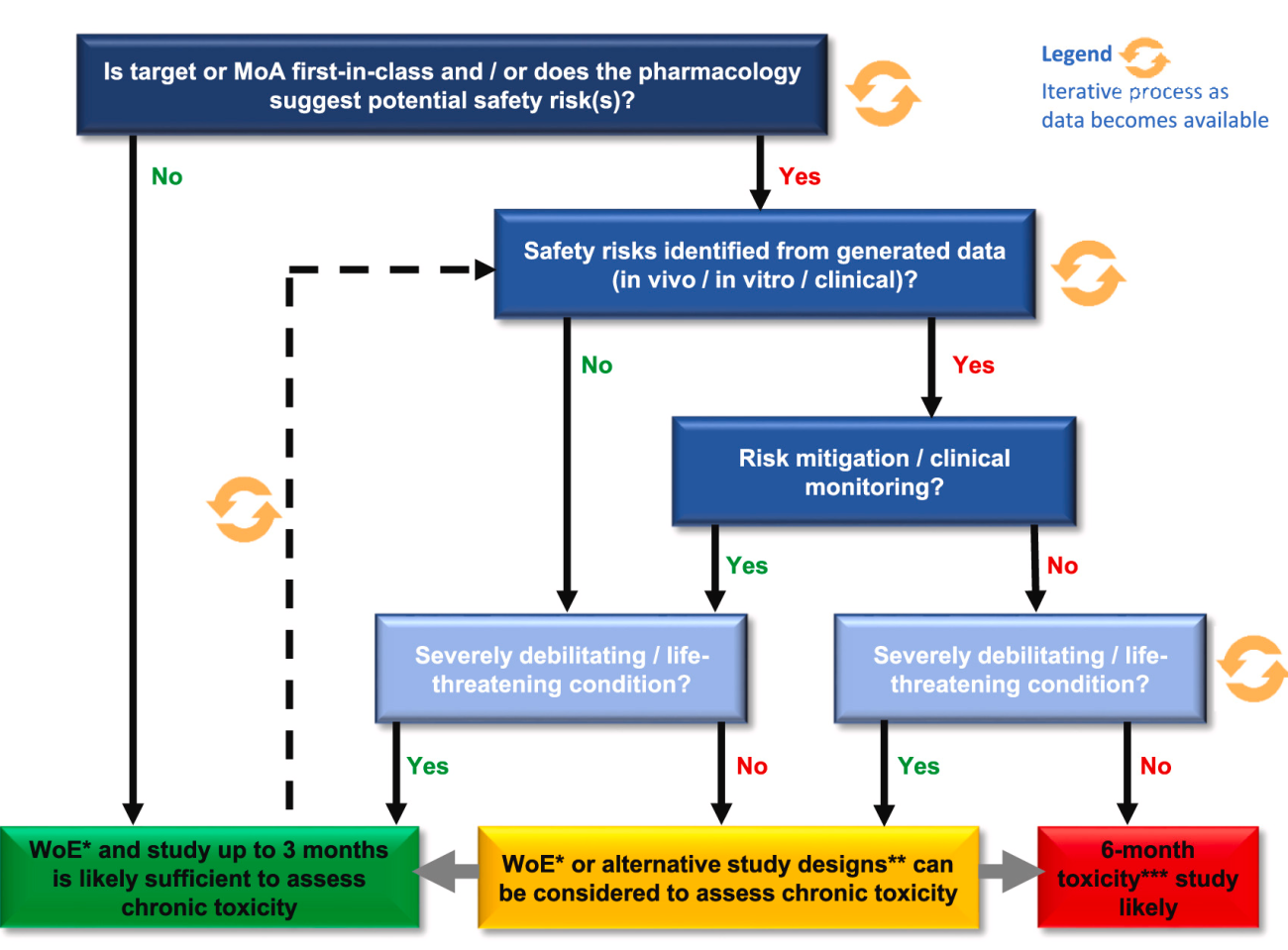
Iterative weight-of-evidence model to determine optimal duration of mAb chronic studies
To support registration of monoclonal antibodies (mAbs) for chronic indications, 6-month toxicity studies have historically been conducted. Experience with mAb development has shown a relatively benign and well-understood safety profile for this class, with most toxicity findings anticipated based on pharmacology.
We evaluated whether a 6-month toxicity study is necessary to assess the long-term safety of mAbs.
Data on First-in-Human (FIH)-enabling and chronic toxicity studies were shared for 142 mAbs submitted by 11 companies. Opportunities to further optimize study designs to reduce animal usage were identified. For 71% of mAbs, no toxicities or no new toxicities were noted in chronic studies compared to FIH-enabling study findings. New toxicities of potential concern for human safety or that changed trial design were identified in 13.5% of cases, with 7% being considered critical and 2% leading to program termination.
An iterative, weight-of-evidence (WoE) model which considers factors that influence the overall risk for a mAb to cause toxicity was developed. This model enables an evidence-based justification, suggesting when 3-month toxicity studies are likely sufficient to support late-stage clinical development and registration for some mAbs.
Figure: Iterative WoE decision model developed to support clinical development (modified from [1]).
Optimal strategies within the mAb dataset highlights 3Rs opportunities
- Using one pharmacologically-responsive species, or reducing from two to one (preferably rodent) for long-term studies.
- A minimum package (one study supporting FIH + one study for later development).
- Two test article-dosed groups instead of the standard three groups.
- Increasing the group size for long-term studies is not necessarily required: 3M + 3F (non-rodents) and 10M + 10F (rodents) is an adequate main test group size for all study durations.
- Restrict recovery animal use to long-term studies and only if scientifically justified, not default:
- Make use of scientific assessment (experience, literature) instead of recovery animals.
- If warranted, consider recovery in only one species and sex where appropriate.
- If warranted, minimise recovery group numbers (no controls, one dose) and group sizes (2M + 2F non-rodents; 5M + 5F rodents).
Use of recovery animal during mAb development
Assessment of reversibility from nonclinical toxicity findings in animals with potential adverse clinical impact is required during pharmaceutical development, but there is flexibility around how and when this is performed and if recovery animals are necessary. For monoclonal antibodies (mAbs) and in accordance with ICH S6(R1) if inclusion of recovery animals is warranted, this need only occur in one study.
The data on study designs for first-in-human (FIH)-enabling and later-development toxicity studies described above were shared from a recent collaboration between the NC3Rs, EPAA, Netherlands Medicines Evaluation Board (MEB) and 14 pharmaceutical companies. This enabled a review of practices on recovery animal use during mAb development.
Recovery animals were included in 68% of FIH-enabling and 69% of later-development studies, often in multiple studies in the same program. Recovery groups were commonly in control plus one test article-dosed group or in all dose groups (45% of studies, each design).
Based on the shared data review and conclusions, limiting inclusion of recovery to a single nonclinical toxicology study and species provide opportunities to further reduce animal use within mAb development programs.
You can read about our previous work on this topic on our project page: Reducing the use of recovery animals.
Publications
-
Chien H et al. (2023). Re-evaluating the need for chronic toxicity studies with therapeutic monoclonal antibodies, using a weight of evidence approach. Regulatory Toxicology and Pharmacology 138: 105329. doi:10.1016/j.yrtph.2022.105329.
-
Prior H et al. (2023). The use of recovery animals in nonclinical safety assessment studies with monoclonal antibodies: further 3Rs opportunities remain. Regulatory Toxicology and Pharmacology 138: 105339. doi:10.1016/j.yrtph.2023.105339.
-
Prior H et al. (2022). The Use of Recovery Animals across Monoclonal Antibody Development Packages: Opportunity for Further Optimization Remains (PDF, 191KB). The Toxicologist (Supplement to Toxicological Sciences) 186: P401-402.
-
Chien H et al. (2022). Evaluating Optimal Toxicity Study Designs with Monoclonal Antibodies: Results from a MEB/Industry/NC3Rs Survey (PDF, 1.7MB). The Toxicologist (Supplement to Toxicological Sciences) 186: P402.
-
Chien H et al. (2022). Re-evaluating the need for chronic toxicity studies with therapeutic monoclonal antibodies, using a weight of evidence approach (PDF, 2.0MB). Toxicology Letters 368 (S1): S260-261. doi:10.1016/j.toxlet.2022.07.691.
